Reviewed by Lexie CornerMay 30 2025
How do people think, feel, remember, and move? These processes rely on synaptic transmission, where chemical signals are passed between nerve cells using molecular structures called vesicles. Researchers have now reconstructed the vesicle cycle in greater detail, providing new insights into brain function.
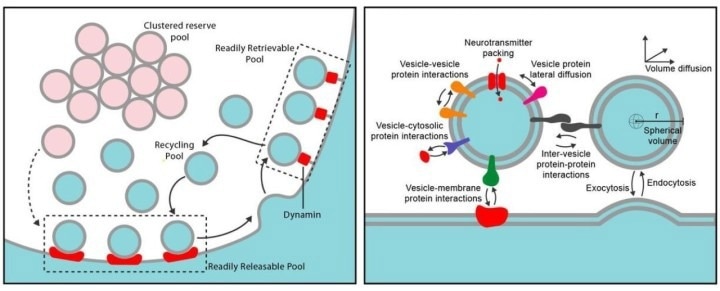 Left) The different pools involved in the vesicle cycle. Vesicles dock to form a readily releasable pool. At the membrane surface, vesicles can be rapidly endocytosed by dynamin to form, in addition, a readily retrievable pool. Right) The wide variety of interactions is accounted for within the researcher’s model. Image Credit: Gallimore et al.
Left) The different pools involved in the vesicle cycle. Vesicles dock to form a readily releasable pool. At the membrane surface, vesicles can be rapidly endocytosed by dynamin to form, in addition, a readily retrievable pool. Right) The wide variety of interactions is accounted for within the researcher’s model. Image Credit: Gallimore et al.
Scientists from the Okinawa Institute of Science and Technology (OIST) in Japan and the University Medical Center Göttingen (UMG) in Germany collaborated on this study, which was published in Science Advances. They developed a computational model that accounts for the complex interactions, functions, and cellular environments involved in vesicle activity. This results in a more detailed representation of how vesicles contribute to synaptic transmission.
The model also predicts aspects of synaptic function that have been difficult to measure directly, potentially informing future research in neuroscience.
Recent technological advances have enabled experimental scientists to capture increasing amounts of data. The challenge now lies in integrating and interpreting all the different types of data to understand the complexities of the brain. Our model provides better molecular and spatial detail of the vesicle cycle, and is much faster than any other system before. And it is transferable to different cells and scenarios, too. It is a significant leap forward towards scientific aspirations of full cell and full tissue simulation.
Erik De Schutter, Study Co-Author and Professor, Computational Neuroscience Unit, Okinawa Institute of Science and Technology
“We have been working on synapses for over 20 years, but some functional steps were difficult to test experimentally. After several years of fine-tuning experimental and computational work with our Japanese colleagues, we now have a model for testing new hypotheses, especially in the context of neurological diseases,” added Professor Silvio Rizzoli, director of the Department for Neuro and Sensory Physiology at the UMG and also co-author on the study.
What Exactly is the Synaptic Vesicle Cycle?
The vesicle cycle describes how neurotransmitters (chemical signals) are released at synapses, the junctions between nerve cells, to transmit information. Vesicles containing neurotransmitters move toward and dock at the cell membrane, where they prepare to fuse and release their contents. After release, the vesicles are recycled. This process is initiated by electrical signals in the brain and driven by a series of molecular signaling events.
The release of neurotransmitters must be precisely regulated, varying in amount and timing depending on the situation. Only about 10–20 % of vesicles are typically positioned for immediate docking, forming what is known as the recycling pool, which supports sustained transmission. The remaining vesicles are held in a reserve pool, where they remain inactive under normal conditions.
Many aspects of this process, such as how vesicles migrate between the reserve and recycling pools, were unknown.
The Mechanisms of Vesicle Recycling at High Stimulation Frequency
The researchers investigated vesicle recycling in hippocampal synapses, using their model to validate vesicle behavior at experimentally observed firing frequencies and to examine responses under higher-frequency stimulation.
Their results showed that the vesicle cycle can function at stimulation frequencies well above typical physiological levels. They also identified factors contributing to the cycle's resilience, including the roles of two proteins - synapsin-1 and tomosyn-1 - in regulating the release of vesicles from the reserve pool.
The study found that molecular tethering plays an important role in maintaining efficient vesicle cycling. Anchoring specific vesicles to the membrane helped make more vesicles available for rapid docking and neurotransmitter release.
These findings provide new insights into the mechanisms of vesicle recycling, which are relevant to the study of various neurological conditions.
De Schutter concluded, “For example, the release of neurotransmitters is hampered in botulism or some myasthenic syndromes. Treatments for depression and other major neurological diseases also often focus on synaptic transmission. As we expand our models, the potential applications are vast, both in developing new therapeutics and in deepening our fundamental understanding of how the brain works.”
Source:
Journal reference:
Gallimore, A. R. et al. (2025) Dynamic regulation of vesicle pools in a detailed spatial model of the complete synaptic vesicle cycle. Science Advances. doi.org/10.1126/sciadv.adq6477