 By Pooja Toshniwal PahariaReviewed by Lauren HardakerNov 20 2025
By Pooja Toshniwal PahariaReviewed by Lauren HardakerNov 20 2025New imaging results reveal that lecanemab’s early effects stop short of improving a key waste-clearance marker, suggesting the brain’s glymphatic system may take longer to respond than amyloid plaques.
 Image credit: Only_NewPhoto/Shutterstock.com
Image credit: Only_NewPhoto/Shutterstock.com
Emerging therapies that target amyloid plaques, such as lecanemab, have shown promise in slowing cognitive decline in Alzheimer’s disease (AD). However, new imaging findings suggest their benefits may not extend to the brain’s waste-clearance system early in therapy.
A preliminary study published in the Journal of Magnetic Resonance Imaging reported no significant change in the Diffusion Tensor Imaging Along Perivascular Space (DTI-ALPS) index after three months of treatment. While lecanemab is known from larger trials to reduce amyloid burden, this study did not assess plaque change in its participants.
Since the DTI-ALPS index is considered a surrogate marker suggested to be associated with glymphatic activity, these results hint that underlying glymphatic dysfunction may not improve early in therapy. The findings raise important questions about how quickly therapies can restore brain clearance pathways in AD.
Why Glymphatic Function Matters
AD is driven by the gradual deposition of amyloid aggregates within brain tissue, leading to progressive memory loss and mental slowing. Emerging research suggests that dysfunction in the brain’s waste-clearance system, known as the glymphatic network, is a significant contributor to disease progression. Newly authorized disease-modifying antibody treatments, e.g., lecanemab, aim to slow this deterioration by promoting the removal of amyloid proteins.
The DTI-ALPS index is a non-invasive indicator linked to this clearance activity, typically declining with age and showing markedly lower values in AD patients. However, studies tracking changes in the DTI-based glymphatic activity index after initiating these therapies are lacking, leaving critical questions about their broader impact unanswered.
OASIS and Patient Scans
In the present study, researchers explored whether amyloid-directed therapy influences the brain’s waste-clearance system. To do so, they measured the DTI-based glymphatic activity index in AD patients before and three months after commencing lecanemab therapy. They aimed to provide early reference values to incorporate in future studies monitoring long-term changes in treated patients.
The observational study followed a two-phase approach. Initially, the team analyzed publicly accessible Open Access Series of Imaging Studies (OASIS) data from 23 cognitively healthy volunteers who had undergone two DTI scans acquired with identical parameters. The method allowed assessment of natural variability in the DTI-ALPS index and informed the sample size for subsequent analyses.
Next, the researchers recruited participants at their institution between December 1, 2023, and February 28, 2025. All participants had a confirmed AD diagnosis by a neurologist, underwent magnetic resonance imaging (MRI) during lecanemab screening, and formally consented in writing.
The team performed brain imaging on a 3T system using a standardized single-shot echo-planar DTI protocol with a 20-element head coil. They conducted baseline scans within three months prior to the initial lecanemab dose, followed by a follow-up scan exactly three months after treatment onset.
Before evaluation, the researchers carefully pre-processed all DTI data to reduce noise, remove artifacts, correct for motion artifacts and distortions, and adjust for intensity variations. They then used automated methods to reorient DTI vectors and register images across time points. They defined regions of interest (ROIs) in both hemispheres, and averaged the mean diffusivity along three axes within these ROIs to generate a DTI-ALPS value for each participant. The approach allowed the team to track changes in this surrogate marker of glymphatic activity following therapy.
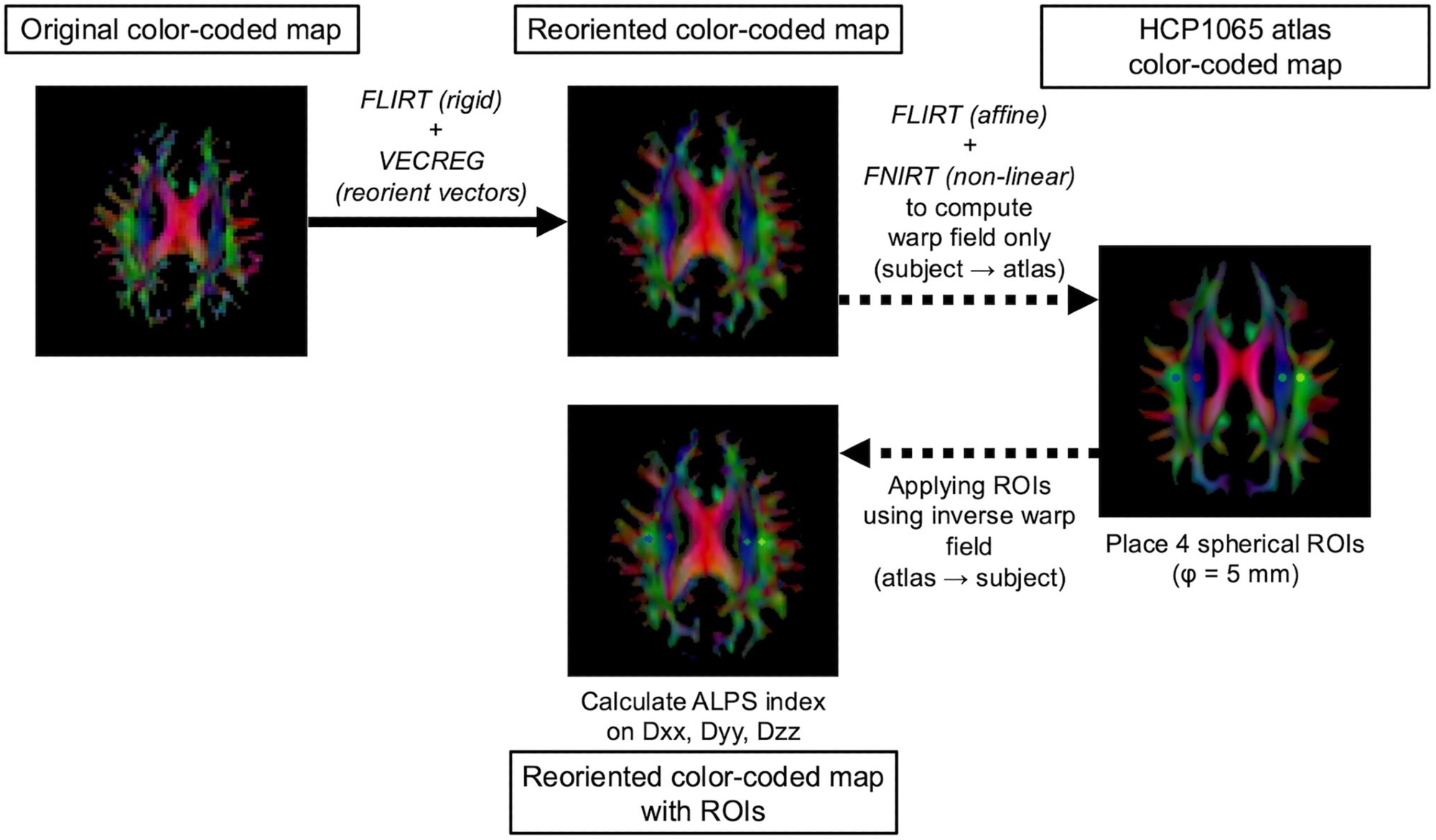 Workflow for automated DTI-ALPS index calculation. DTI data are visualized as color-coded direction maps for illustration purposes.
Workflow for automated DTI-ALPS index calculation. DTI data are visualized as color-coded direction maps for illustration purposes.
Early Effects Remain Limited
Of the 32 AD patients receiving amyloid-directed therapy, 13 were eligible for analysis. Participants had a mean age of 72 years, including five men and eight women. The mean baseline DTI-ALPS metric was 1.515, which remained virtually unchanged at 1.513 after three months of lecanemab therapy. Statistical analysis revealed no statistically significant difference between baseline and follow-up measurements, indicating that DTI-ALPS values remained unchanged during the initial treatment period. Using an equivalence-testing approach with a predefined margin of ±0.05, it was also demonstrated that any possible treatment-related change in the DTI-ALPS index over three months was likely to be very small.
Although the sample size was small, it was specifically power-estimated, offering an early reference point for interpreting DTI-derived glymphatic activity indices in future AD studies. The findings suggest that, while amyloid-focused therapies may reduce plaque accumulation, the diffusion characteristics of perivascular regions, captured by the DTI-ALPS surrogate marker, did not improve within the first three months of treatment.
Overall, the study findings demonstrate that early treatment with lecanemab did not result in measurable changes in the DTI-ALPS index over a three-month period. The observations suggest that short-term therapy may not immediately restore perivascular space function or glymphatic clearance, reflecting the multifactorial nature of AD, where neuronal injury and impaired clearance mechanisms are not easily reversible. It is also cautioned that DTI-ALPS may be influenced by white-matter geometry and anatomical factors, meaning it should not be interpreted as a direct measure of glymphatic function.
While this small, power-estimated cohort provides an initial benchmark, future studies should include larger populations, longer follow-up, and other plaque-reducing therapies, e.g., donanemab. Careful control of anatomical and technical variables will also be essential to investigate whether prolonged therapy can influence perivascular diffusion and brain waste clearance.
Download your PDF copy now!
Journal Reference
Oura, T. et al. (2025). Unchanged Early Diffusion Tensor Imaging Along Perivascular Space Index After Amyloid-Targeting Disease-Modifying Therapy in Alzheimer's Disease: A Preliminary Study. Journal of Magnetic Resonance Imaging, 62(6), 1892-1894. DOI: 10.1002/jmri.70118. https://onlinelibrary.wiley.com/doi/10.1002/jmri.70118