Reviewed by Lexie CornerJun 2 2025
The pyruvate dehydrogenase complex (PDHc) plays a key role in metabolism by linking glycolysis to the tricarboxylic acid cycle and converting pyruvate into acetyl-CoA. However, due to its large size and complex structure, the detailed architecture of PDHc is still not well understood.
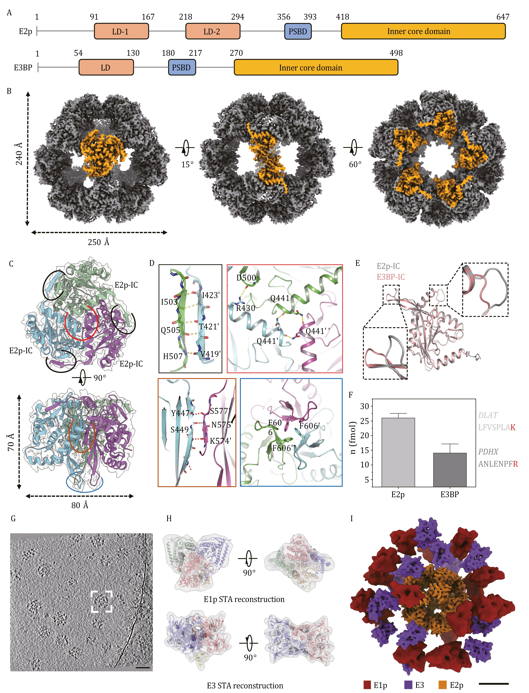 Structural characterization of Sus scrofa PDHc core and overall landscape of integrated complex. Image Credit: TranSpread
Structural characterization of Sus scrofa PDHc core and overall landscape of integrated complex. Image Credit: TranSpread
Early models suggested a highly organized PDHc structure, but the arrangement and behavior of peripheral subunits remained unclear. This lack of structural detail has made it difficult to understand PDHc-related conditions such as lactic acidosis and certain neurological disorders, highlighting the need for high-resolution imaging of the complex in its natural state.
A recent study, published on August 24, 2024, in Protein & Cell by researchers from Tsinghua University, Shenzhen University, King Abdullah University of Science and Technology (KAUST), and others, addressed this challenge using advanced cryo-electron microscopy (cryo-EM) and cryo-electron tomography (cryo-ET) to examine PDHc extracted from porcine heart tissue.
The team achieved near-atomic resolution (3.66 Å) of the inner core and captured the dynamic behavior of the peripheral enzymes E1p and E3, providing new insights into the structure and function of the complex.
They found that the core consists of a dodecahedral scaffold made up of 60 inner core domains. Using quantitative isotopic mass spectrometry, the study confirmed the long-debated "40:20" ratio of E2p to E3BP subunits.
Cryo-ET imaging revealed that the peripheral E1p and E3 subunits are not fixed in a rigid shell but instead form a flexible, asymmetrical layer around the core. On average, each complex contained 21 E1p and 13 E3 subunits, distributed in a pattern that followed a Gaussian curve.
The study also identified previously unknown interaction patterns between E1p and the lipoyl domains of E2p, suggesting a potential regulatory mechanism that could adjust PDHc activity in response to metabolic changes.
This study overturns decades of structural assumptions. We now understand that PDHc’s apparent disorder is, in fact, a design feature, allowing it to rapidly adjust to metabolic demands. This flexible architecture may be the key to its efficiency, and a critical factor in disease pathology when the system breaks down.
Dr. Sai Li, Study Co-Corresponding Author, Tsinghua University
These findings may support the development of new treatment strategies for disorders related to PDHc, including inherited metabolic conditions and mitochondrial dysfunction. Identifying flexible interaction sites within the complex could help guide the design of compounds that selectively modulate its activity.
In addition, the integrative imaging approach used in this study offers a valuable method for studying large, dynamic protein assemblies in their native state. This could advance research in structural biology, metabolic engineering, and drug development.
Source:
Journal reference:
Chen, M., et al. (2024) Molecular architecture of mammalian pyruvate dehydrogenase complex. Protein & Cell. doi.org/10.1093/procel/pwae044.