 By Pooja Toshniwal PahariaReviewed by Lauren HardakerSep 3 2025
By Pooja Toshniwal PahariaReviewed by Lauren HardakerSep 3 2025In a recent study published in Frontiers in Bioengineering and Biotechnology, researchers developed a human-derived three-dimensional (3D) wounded skin equivalent (3DWoundSE) model replicating early wound-healing events, including barrier disruption, cell death, proliferation, and inflammation.
 Image credit: Oleksandr Yuhlichek/Shutterstock.com
Image credit: Oleksandr Yuhlichek/Shutterstock.com
Although part of the higher damage signals reflect stress from the wounding procedure itself, the 3DWoundSE still demonstrated greater responsiveness than intact 3D skin. It is a reproducible and ethically sound platform for studying wound dynamics and testing therapies, reducing reliance on animal models.
Current wound-healing models often fail to reproduce human skin physiology. Two-dimensional cultures lack critical cell–cell interactions, extracellular matrix organization, and nutrient gradients. In contrast, animal models diverge in structure, immune responses, and repair mechanisms, which limits their translational relevance and raises ethical concerns. Ex vivo human skin closely reflects natural structure, but limited availability, variability, and short viability restrict its use. Three-dimensional skin models overcome these limitations by mimicking native architecture and providing a reproducible, human-relevant, and ethically sound platform for wound-healing research.
About The Study
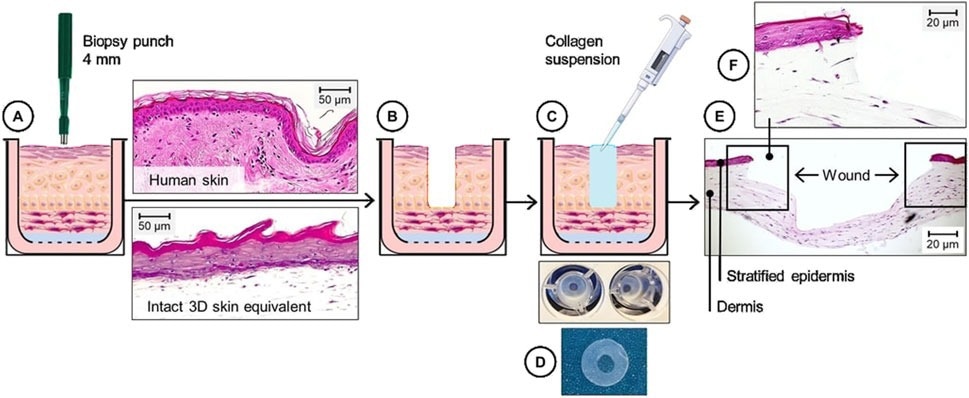
In the current study, researchers engineered 3DWoundSE, a 3D human-derived wounded skin model, as a physiologically relevant platform for studying wound-healing responses in vitro. They established the model by applying a controlled partial-thickness punch wound to a previously matured three-dimensional skin equivalent (3DSE) model.
To construct the 3DSE, the researchers embedded human skin fibroblasts (3.5 × 10⁴) in rat collagen gel (8.8 mg/mL) in 12 culture wells. They incubated the cells in a fibroblast cell culture medium and coated the gel surface with fibronectin to promote keratinocyte adhesion. Subsequently, they seeded 3.2 × 10⁵ human epidermal keratinocytes (HEKs) on top for proliferation in the growth medium. After 72 hours, they elevated the structures to the air-liquid boundary and allowed cell differentiation over 26 days, changing the culture media every three days. The process yielded a stratified and organotypic three-dimensional model of human skin.
On day 30 of culture, the team created a central dermal defect in the 3DSE, measuring 4.0 mm in diameter, via punch biopsy. Subsequently, they filled the defect with collagen gel to preserve structural stability.
The model underwent histological and immunofluorescence analyses at 24, 48, and 72 hours post-wounding to assess tissue structure and cellular dynamics. Staining with markers such as apoptosis-inducing factor (AIF), E-cadherin, and Ki-67 enabled the evaluation of apoptosis, proliferation, and cell–cell adhesion, respectively. Fluorescence microscopy determined stain localization and intensity.
Lactate dehydrogenase (LDH) assays revealed cytotoxicity based on membrane integrity and cell death. The authors note that LDH in this model reflects post-wounding stress rather than absolute cytotoxicity. The expression of cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukins (ILs)-1α, 6, 8, and 33 in the culture supernatants indicated the extent of inflammation. To validate the 3DSE's physiological relevance, the researchers referred to histological comparisons with ex vivo human skin from an elderly female hand, which had been detailed in their prior work.
 Study: Engineering a 3D wounded skin equivalent to study early inflammatory and regenerative responses in vitro
Study: Engineering a 3D wounded skin equivalent to study early inflammatory and regenerative responses in vitro
Results
3DSE successfully formed a stratified epidermis with a dense dermal compartment resembling native human skin. In contrast, 3DWoundSE displayed complete epidermal removal extending into the dermis, with wound margins comprising completely stratified dermal layers. Structural sensitivity assays revealed that 3DWoundSE was more susceptible to disruption by Triton detergent than 3DSE, a finding further supported by elevated LDH levels, indicating increased cell death in barrier-compromised skin.
Molecular analyses confirmed distinct injury responses in the 3D skin equivalent model. The team found significantly elevated AIF expression 24 hours post-wounding, reflecting enhanced apoptotic activity. However, levels declined by 48 hours while remaining above 3DSE levels. Similarly, LDH assays demonstrated sustained cytotoxicity in wounded constructs.
Proliferative activity, assessed by Ki-67 staining, decreased immediately after wounding but rebounded at 48 hours, with increased proliferation localized to basal and suprabasal layers near the wound edge, evidence of re-epithelialization. E-cadherin expression, a marker of cell–cell adhesion, was slightly reduced in the early phase, though not statistically significant. This is consistent with epithelial disruption from wounding, which began to recover during the early healing phase.
The team found clear differences in inflammatory responses between the models. Compared to 3DSE, the 3DWoundSE exhibited elevated secretion of inflammatory cytokines, including TNF-α and ILs-6, 8, and 33. Notably, IL-33, an epithelial alarmin released upon cellular stress, was significantly increased, consistent with the mechanical injury to epithelial layers. This cytokine profile highlights the model’s ability to simulate key early inflammatory events following wounding.
Conclusions
Based on the findings, 3DWoundSE successfully reflects key aspects of early wound healing, offering a reproducible and ethically responsible alternative to animal models for investigating wound dynamics and evaluating therapeutic strategies. The 3D skin model provides a versatile foundation for dermatological research, drug discovery, and preclinical testing.
While the current model relies on rat-derived collagen and lacks skin appendages such as hair follicles and sebaceous glands, integrating these features could improve clinical relevance. Future improvements, including the addition of immune and vascular components, disease-specific adaptations like diabetic skin models, bacterial inoculation for infection studies, and microfluidic platforms, hold promise for capturing systemic factors in wound repair.
Download your PDF copy now!
Journal Reference
Nuwayhid R, Ngoc-Huyen N, Notov D, Langer S, and Kurow O (2025). Engineering a 3D wounded skin equivalent to study early inflammatory and regenerative responses in vitro. Front. Bioeng. Biotechnol., 13:1621566. DOI: 10.3389/fbioe.2025.1621566 https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1621566/full