Reviewed by Lauren HardakerSep 8 2025
This review article focuses on the transformative potential of in vivo CAR T cell treatment in overcoming the constraints of standard CAR T cell production. This novel strategy might transform cancer therapy by providing a more efficient, scalable, and cost-effective alternative to traditional procedures.
 Image credit: Nemes Laszlo/Shutterstock.com
Image credit: Nemes Laszlo/Shutterstock.com
CAR T cell therapy has demonstrated exceptional efficacy in treating hematological malignancies; nevertheless, existing manufacturing processes are tedious, time-consuming, and costly.
Traditional in vitro CAR T cell manufacturing takes 2-3 weeks and involves several complex steps, including T cell separation, activation, genetic alteration, expansion, and quality control. This time-consuming process is compounded further by the requirement for individualized manufacture, which limits its use in quickly advancing diseases.
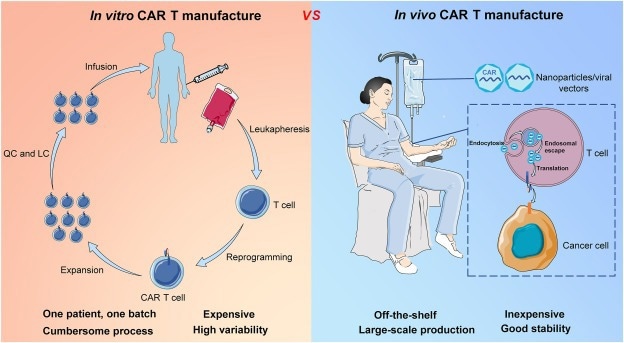
The comparison of in vitro and in vivo CAR T cell therapy. Image credit: Song, Z., et al. (2025)
The in vivo technique constitutes a breakthrough since it eliminates the need for considerable laboratory manipulation. Instead of producing CAR T cells outside the body, this strategy includes directly delivering CAR constructs to T cells within the patient's body. The procedure uses viral and nonviral vectors to modify T cells genetically, allowing them to target and kill cancer cells effectively.
One of the primary benefits of in vivo CAR T cell manufacturing is its capacity to scale and lower expenses. In contrast to the “one patient, one batch” approach of in vitro procedures, in vivo techniques can produce “off-the-shelf” CAR T cell products, enabling large-scale manufacturing and greater accessibility. Furthermore, this technique retains T cell functioning, increasing therapeutic effectiveness as compared to in vitro-produced CAR T cells, which frequently undergo functional degradation.
The research emphasizes that in vivo CAR T cell treatment is especially promising for quickly developing malignancies due to its short reaction time. Furthermore, using nanoparticle-based technologies and viral vectors such as lentiviral (LV) and adeno-associated virus (AAV) provides effective gene transfer and long-term CAR expression. These vectors have shown greater transfection rates and negligible safety hazards compared to previous approaches.
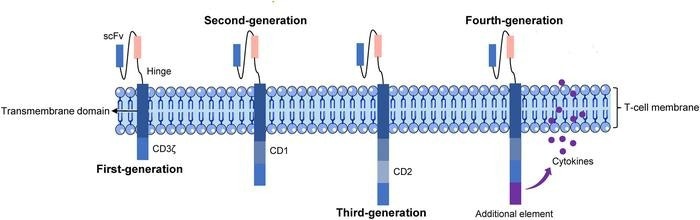 The basic structure of five generations of CAR T cells. Image Credit: Genes & Diseases
The basic structure of five generations of CAR T cells. Image Credit: Genes & Diseases
However, the strategy is not without its problems. The possibility of off-target effects, immunogenicity, and the danger of insertional mutations remains an essential field of study. Addressing these problems is critical for the wider clinical use of in vivo CAR T cell treatments. Furthermore, balancing cost-effectiveness with high transfection efficiency will decide the technique's practical feasibility.
Source:
Journal reference:
Song, Z., et al. (2025) In vivo production of CAR T cell: Opportunities and challenges. Genes & Diseases. doi.org/10.1016/j.gendis.2025.101612