Reviewed by Lauren HardakerFeb 4 2026
A novel gel might help combat resistant bacteria in wounds and around implant sites while also promoting recovery. The hydrogel, inspired by natural immune defenses, has shown extremely encouraging results in animal models.
 Image credit: DeawSS/Shutterstock.com
Image credit: DeawSS/Shutterstock.com
Approximately 7.7 million fatalities globally are caused by bacterial infections each year, and the issue is made worse by growing antibiotic resistance. In addition to being more challenging to treat, wound infections also prevent the surrounding tissue from recovering.
This is due to a misdirected inflammatory response caused by the wound infection, which destroys healthy tissue and obstructs the healing processes. Even if they are efficient against the underlying germs, antibiotics are not very helpful in such circumstances.
How Our Immune Cells’ Protein Nets Work
This serves as the foundation for a novel strategy developed at ETH Zurich and published in Nature Communications.
Their strategy draws inspiration from the net-like protein structures released by immune cells to ensnare and neutralize invaders. As a type of natural snare, these neutrophil extracellular traps (NETs) stop infections from spreading throughout the body.
Artificial imitations of these structures have already been tested. Nevertheless, the synthetic materials employed in earlier experiments proved to be too unstable, too intolerable, or insufficiently effective against resistant bacteria.
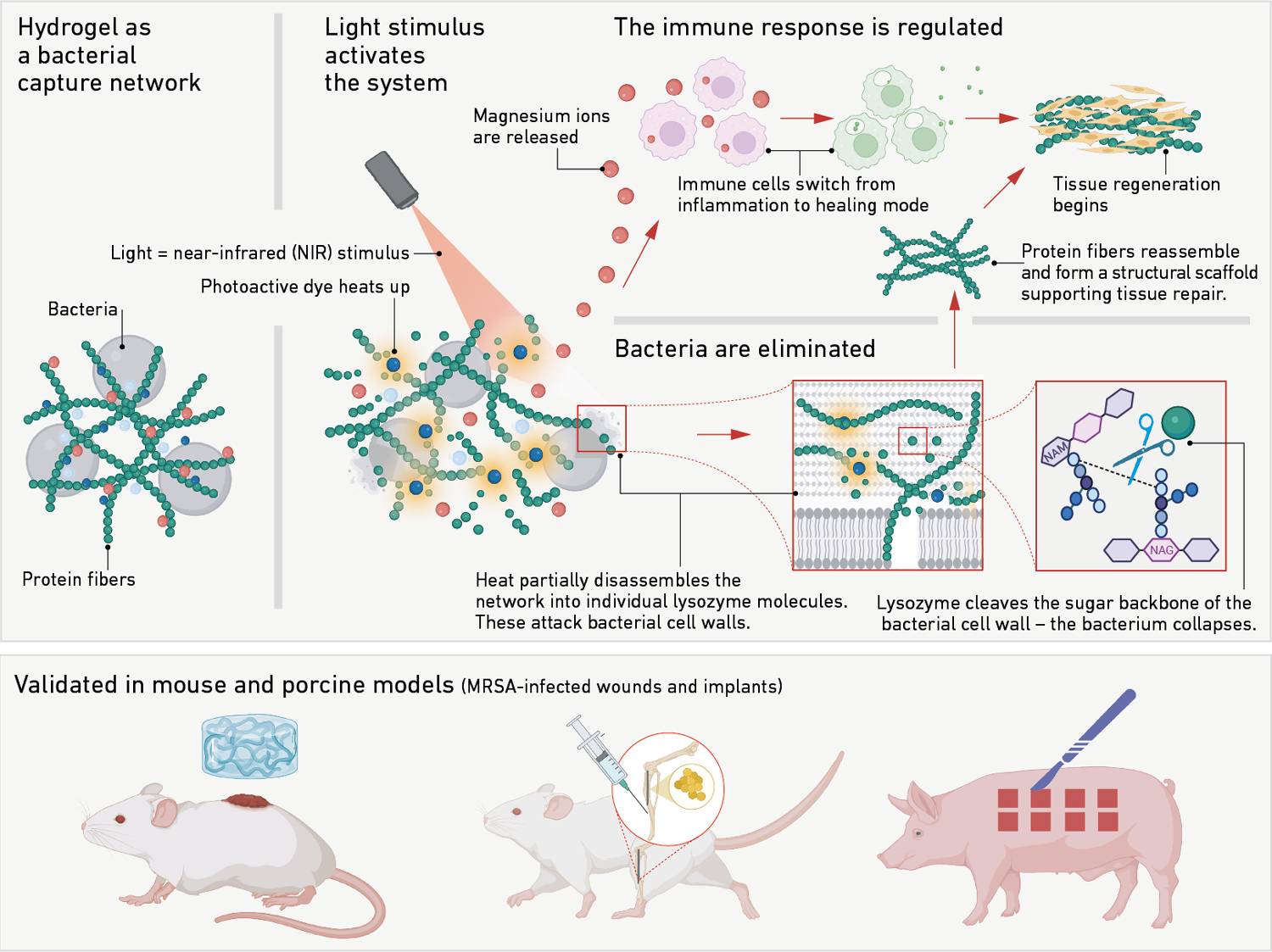 How the hydrogel traps bacteria and promotes wound healing. Image Credit: Xuan Q et al. Nature Communications 2025.
How the hydrogel traps bacteria and promotes wound healing. Image Credit: Xuan Q et al. Nature Communications 2025.
Antibacterial Enzyme Activated by Infrared Light
Unlike many synthetic approaches, we rely on a natural, protein-based system.
Raffaele Mezzenga, Professor, ETH Zurich
The gel is formed from hen's egg whites and consists of a dense mesh of small protein fibers derived from lysozyme, which is inactive in this form. Lysozyme is an antibacterial enzyme that is also found in the human body. The gel functions as a physical net, settling over the wound and containing the bacteria within.
The final step in activating the enzyme is as simple as pressing a button: exposing the gel to near-infrared light, a mild, minimally intrusive approach, warms up a thermally sensitive molecule designed specifically for this purpose.
The heat produced by this molecule causes a portion of the protein fiber net to disintegrate briefly, releasing individual lysozyme molecules. In this state, the lysozyme molecules are biologically active. They assault the bacteria, specifically their cell walls, and eliminate the infection.
Replacing Chronic Inflammation with Healing
In addition, when triggered by light, the gel releases magnesium ions. Rather than providing an antimicrobial impact, these ions soothe the immune system. They transform pro-inflammatory immune cells into pro-regenerative ones. As a result, instead of sustaining an inflammatory response, the cells now actively assist cell repair, promoting rather than inhibiting healing.
When the light pulse ends, the protein fibers recombine to create a stable net. This implies that the gel once again offers a framework for cell stability while also promoting tissue regeneration.
The hydrogel's primary feature is the reversibility of its fibers, which may be activated to deconstruct and reassemble.
Our technology combines antibacterial and anti-inflammatory effects with wound healing. One day, it could open new possibilities, especially for diabetic patients with chronic wounds and for patients battling with antibiotic resistance.
Qize Xuan, Visiting Doctoral Student and Study Lead Author, ETH Zurich
Bacterial Load in Animal Models Reduced by 95 Percent
The hydrogel has previously been tested in preclinical trials on mice and pigs. In the murine model, the gel decreased bacterial load in a wound infected with antibiotic-resistant MRSA by 95 %. Furthermore, the treated wound closed almost completely within 15 days, but the untreated controlled wounds healed much more slowly.
Along with noticeably less bacterial colonization, the porcine model also showed faster wound healing. Furthermore, the material produces an environment that is conducive to the growth of new soft tissue and bone.
When the gel is immediately administered to the wound, it stays there as the wound heals. As the tissue regenerates, it is incorporated into the tissue and progressively biodegrades.
However, there is still a long way to go before patients can use the gel. Clinical trials will be the next stage.
We are now searching for industry partners to assist us. Trials like this are laborious, expensive and only possible in close collaboration with hospitals.
Raffaele Mezzenga, Professor, ETH Zurich
Source:
Journal reference:
Xuan, Q. et.al. (2026) Photo-reversible amyloid nanoNETs for regenerative antimicrobial therapies. Nature Communications. DOI: 10.1038/s41467-025-65976-6. https://www.nature.com/articles/s41467-025-65976-6.