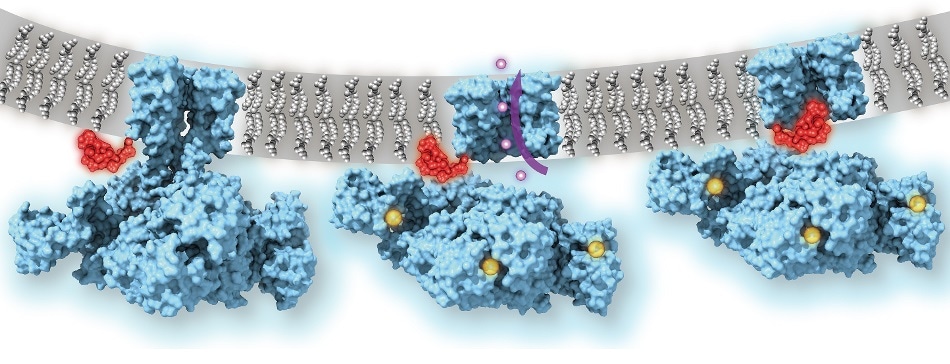
Calcium-gated potassium channel MthK in closed, open, and inactivated states, from left to right. Channel structure (blue), with one subunit removed for clarity; calcium ions (yellow); potassium ions (purple); membrane (grey); N-terminal inactivation peptide (red). The location of the peptide in the inactivated channel was identified in the structural analysis, whereas the location shown in closed and open channels is hypothetical. Image Credit: Dr Crina Nimigean.
Using electron-microscopy techniques to directly visualize the ball-and-chain mechanism offers a new perspective to design drugs targeting it to enhance the function of ion channels.
Abnormalities in ion channels are associated with various types of disorders, such as diabetes, epilepsies, heart arrhythmias, and schizophrenia.
Scientists have been trying to get an atomic-scale picture of this mechanism since the 1970s, and now that we have it at last, it can become an important drug target.”
Dr Crina Nimigean, Study Senior Author and Associate Professor of Physiology and Biophysics, Department of Anesthesiology, Weill Cornell Medicine
Several types of ion channels, such as those essential for neuronal signaling and heart functioning, tend to open up physically, thereby permitting movement of ions in or out of the cell, upon applying specific stimulus.
On the other hand, few ion channels require an additional, on-the-fly mechanism to prevent ion flow—even if stimulus is applied and structure of the channel is open—for turning ion flow on or off with sufficient frequencies to fulfill the requirements of neurons, heart muscle cells, and cells of other types.
Since 1973, based on biochemical experiments, scientists have made assumptions that such an on-the-fly mechanism is similar to a bathtub plug on a chain, or “ball-and-chain” structure. However, it has been highly difficult to confirm this directly with atomic-scale imaging techniques.
The challenge is primarily because of the complex nature of these channels in mammals and the difficulty faced while reconstructing them, for purposes of imaging, in a cell-membrane-like environment in which they are linked to other cell membrane components.
Nobody knew exactly how this process actually looks and works—does the “ball” block the opening of the channel, or actually go in and plug the pore, or alternatively, alter the conformation of the channel indirectly?”
Dr Crina Nimigean, Study Senior Author and Associate Professor of Physiology and Biophysics, Department of Anesthesiology, Weill Cornell Medicine
Nimigean and her team could solve this by visualizing a potassium ion channel from Methanobacterium thermoautotrophicum—a bacteria-like species that occurs at deep-sea geothermal vents.
The “MthK” channel in this species is found to be structurally similar to the mammalian “BK” potassium channel that is essential for the proper functioning of neurons and several other types of cells. However, MthK has major simplifications that render it easier to visualize.
The researchers used low-temperature electron microscopy (cryo-EM), which bounces electrons rather than light off objects to capture atomic-scale images of them, to capture images of the MthK channel when it was switched open by calcium and switched closed.
The images showed that even if the MthK channel is in its calcium-activated “open” state, the path through which ions flow was blocked by a flexible element that clogs the pore of the channel structure.
The researchers ascertained the working of this plug mechanism by demonstrating that when the “ball-and-chain” was genetically deleted, the potassium ion flow through the calcium-activated MthK channel was no longer controlled.
Dr. Nimigean and her team intend to investigate how this mechanism can be targeted therapeutically.
Different classes of potassium channels in human cells are very similar in their channel structures. So a drug that blocks a particular channel will tend to affect other potassium channels and thus could have many unwanted side effects. However, understanding and then targeting this ball-and-chain structure that we were able to image could allow us to therapeutically modulate potassium channels with much more specificity.”
Dr Crina Nimigean, Study Senior Author and Associate Professor of Physiology and Biophysics, Department of Anesthesiology, Weill Cornell Medicine