The metabolic basis of postnatal growth retardation (PGR) in piglets is not yet clear, but it is characterized by poor production performance, low feed conversion rate, and a high mortality rate.
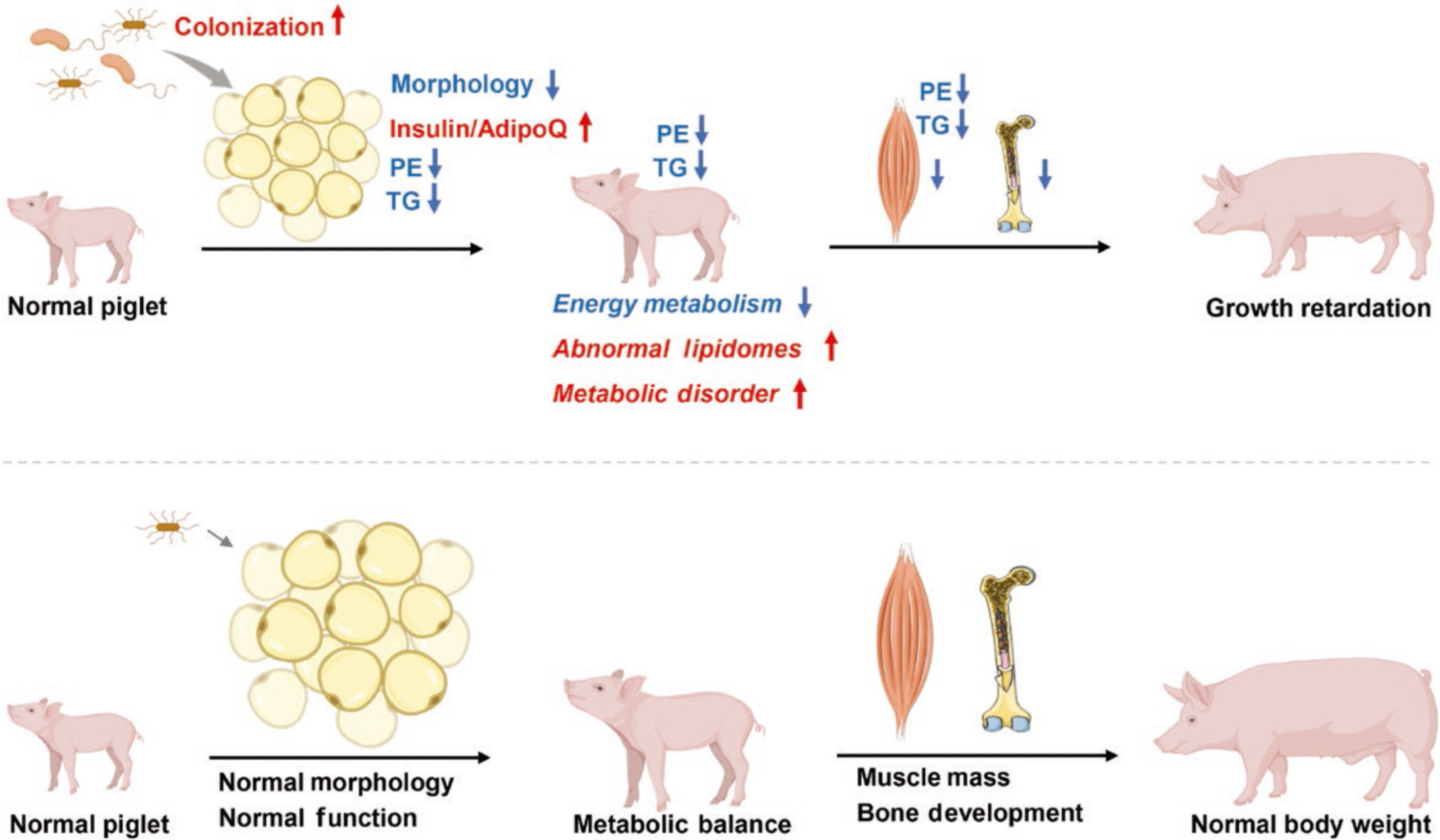 Schematic illustration of PGR-induced S. paucimobilis resided in the adipose tissue. S. paucimobilis residing in the adipose tissue decreases the morphology of the adipose tissue, leading to abnormal lipidomes and metabolic disorders and exacerbating pig growth retardation. Image Credit: Frontiers Journals
Schematic illustration of PGR-induced S. paucimobilis resided in the adipose tissue. S. paucimobilis residing in the adipose tissue decreases the morphology of the adipose tissue, leading to abnormal lipidomes and metabolic disorders and exacerbating pig growth retardation. Image Credit: Frontiers Journals
Adipose tissue, an important organ for energy storage and endocrine regulation, has been found to play a crucial role in regulating glycolipid metabolism and the early growth and development of young animals.
Recent studies have shown that microorganisms in the gut and the environment can migrate to adipose tissue and contribute to the regulation of systemic metabolism. This has been observed in relation to obesity, type 2 diabetes mellitus, and Crohn’s disease. However, it is still unknown whether specific microorganisms exist in PGR pig adipose tissue and if they have any role in regulating growth and development.
Chinese researchers from Huazhong Agricultural University and Hunan Agricultural University recently released a study in Life Metabolism entitled “Disordered microbes from adipose tissue contribute to metabolic issues and worsen postnatal growth retardation in piglets.” This groundbreaking research demonstrates how dysbiosis in adipose tissue disrupts energy metabolism homeostasis, leading to exacerbated PGR in piglets.
The initial step taken by the researchers involved the characterization of PGR models in piglets. By conducting a comprehensive tracking trial and testing various key metabolism-related factors, they were able to observe that PGR piglets exhibited a phenotype resembling insulin resistance. Upon further analysis of their adipose tissue morphology, it was discovered that the diameter of adipose tissue in PGR piglets was significantly smaller compared to that of normal individuals.
This finding suggests that PGR piglets may experience disturbances in glycolipid metabolism.
To investigate the presence of microbes in the adipose tissue and their potential impact on the metabolism of PGR piglets, the researchers collected subcutaneous adipose tissue, visceral adipose tissue, liver, blood, and other tissues from PGR piglets. It was found that the adipose tissues of PGR piglets indeed contained microbes that differed from those found in normal individuals.
Notably, a dysregulated bacterium called Sphingomonas paucimobilis was isolated and identified in the adipose tissue of PGR piglets. To further explore the effects of this bacterium on growth retardation and metabolic disorders, the researchers administered S. paucimobilis to normally growing piglets through gavage, successfully reproducing the observed conditions.
Furthermore, the investigation revealed a significant increase in the expression of extracellular matrix (ECM)-related genes in adipose tissue, indicating that adipose tissue may undergo functional changes in response to bacterial stimulation. Adipose tissue relies on lipids as crucial signals to react to external stimuli.
By utilizing the lipidomic technique, it was demonstrated that the decrease in triglycerides in PGR piglets closely corresponded to the adipose tissue phenotype. Simultaneously, there was a notable decrease in the phospholipid metabolite phosphatidylethanolamine (PE). Subsequent supplementation of PGR piglets with PE confirmed its effectiveness in improving metabolic disorders and ultimately alleviating piglet growth retardation.
This study uncovers that the presence of irregular microorganisms in adipose tissue has an impact on the growth of piglets by influencing the lipid metabolism of adipocytes, consequently disrupting the balance of energy metabolism. These findings offer potential targets for regulating pig growth and development during the initial postnatal phase.
Source:
Journal reference:
Song, T., et al., (2024) Abnormal adipose tissue-derived microbes drive metabolic disorder and exacerbate postnatal growth retardation in piglet. Life Metabolism. doi.org/10.1093/lifemeta/load052