Reviewed by Lauren HardakerMay 22 2025
Proteins produced by RNA segments can aid in the repair of damaged neurons in human bodies. However, in neurological conditions like ALS and spinal muscular atrophy, or after spinal cord injuries, the systems that transport vital RNA to damaged cell sites malfunction. Consequently, damage is irreversible, and RNA molecules are unable to reach their intended location.
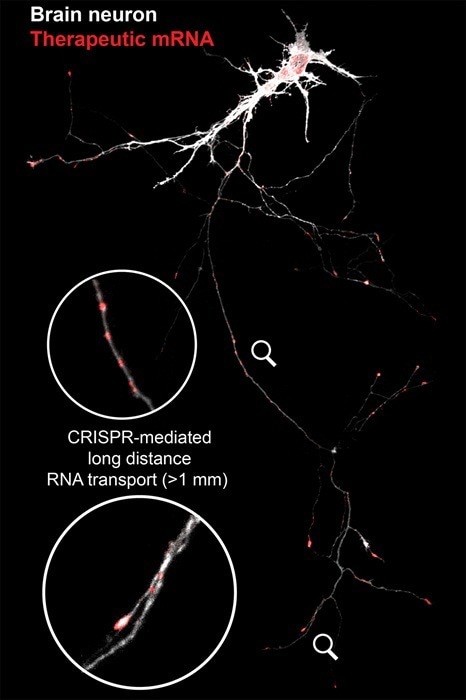 Imaging showing CRISPR-mediated long-distance therapeutic RNA transport for repairing damage in a brain neuron, with the therapeutic RNA tagged red. Image Credit: Mengting Han
Imaging showing CRISPR-mediated long-distance therapeutic RNA transport for repairing damage in a brain neuron, with the therapeutic RNA tagged red. Image Credit: Mengting Han
Stanford researchers have created a method for delivering RNA to particular areas of a neuron, where it can mend and even grow cell components. Supported by the National Institutes of Health, their work lays the groundwork for a new class of therapeutics called “spatial RNA medicine,” which they believe will result in treatments for traumatic injuries and neurological disorders.
For the first time, we have harnessed the power of CRISPR technology to create a precise spatial ‘zip code’ that delivers RNA molecules exactly where they are needed within cells. Imagine being able to specifically target damaged sites within a neuron, repairing them, and promoting their regrowth this is what our technology achieves.
Stanley Qi, Associate Professor and Study Senior Author, Bioengineering, Stanford University
A CRISPR-Based Mailman
Researchers have recently concluded that the location of particular molecules within a cell, or the distribution of RNA, may be just as significant as their functionalities. An individual neuron may extend over a meter in length, and its ability to transport tiny RNA molecules across this distance can be compromised by aging, injury, or genetic mutations.
Qi stated, “Therapeutic RNA cannot help if it does not get to where it is needed. We wanted to create a technology that could reliably move RNA to where it needs to function.”
Unlike the more well-known CRISPR-Cas9, which targets DNA, Qi and his colleagues employed CRISPR-Cas13, a variant of the gene-editing tool CRISPR, to target individual RNA fragments.
Although CRISPR is usually used to cut and modify genetic code, the researchers in this instance were not interested in altering anything. All they wanted to do was relocate the existing RNA to a different location inside the cell.
Qi explained, “Cas13 naturally acts like a pair of scissors, but we engineered it to act like a mailman instead. Then we can instruct it to transport the RNA from one precise location to another.”
The researchers combined Cas13 with specific localization signals that function as addresses, guiding Cas13 to where to deliver the RNA. Each cellular location has its address molecule, allowing the researchers to direct RNA to various sites by incorporating different molecules into the cell.
Using their technology, CRISPR-TO, the researchers screened numerous RNA segments to identify those that could promote neuronal growth.
They applied CRISPR-TO to mouse brain neurons in a petri dish, where it transported RNA molecules to the tips of neurites, fingerlike extensions that form synapses and connect with other neurons. They discovered several promising candidates, including one RNA molecule that enhanced neurite growth by as much as 50 % over 24 hours.
We are discovering more RNA targets that could promote neurite outgrowth and regeneration. We have added a new tool to the CRISPR toolbox, using it to control RNA localization inside the cell. This has never been achieved before and, importantly, it opens new therapeutic directions for treating neurodegenerative diseases.
Mengting Han, Postdoctoral Scholar and Study Lead Author, Stanford University
Safer, More Effective RNA Medicine
The researchers are using CRISPR-TO to screen additional RNA molecules to determine which will be most effective in repairing injured neurons in mouse brains, as well as in human neurons.
Qi remarked, “We are at the beginning of understanding how spatial organization of RNA benefits brain repair. We hope our technology will help people figure out which RNAs will be the biggest players for better therapeutics.”
Currently, the researchers are utilizing CRISPR-TO to move endogenous RNAs (RNA molecules that are naturally produced within the cell). However, according to Qi, it could also be applied to provide precise control over RNA-based medicines, enhancing their safety and efficiency.
This potential excites us tremendously. It is not enough for a molecule to just be in the cell. We need it to be in the right location at the right time. With our precise, programmable technology, you can target any RNA in any type of cell and bring it to the site of need in the body.
Stanley Qi, Associate Professor and Study Senior Author, Bioengineering, Stanford University
Source:
Journal reference:
Scherer, M., et al. (2025) Clonal tracing with somatic epimutations reveals dynamics of blood ageing. Nature. doi.org/10.1038/s41586-025-09041-8.