Reviewed by Lauren HardakerFeb 2 2026
Although DNA is tightly packed and safeguarded within the cell nucleus, it remains continually at risk of damage from normal metabolic activity and external stressors such as radiation and chemical agents. To address this threat, cells depend on a complex network of repair mechanisms. When these systems malfunction, DNA damage can build up, disrupting cellular function and contributing to cancer, aging, and degenerative disorders.
 Image credit: Vink Fan/Shutterstock.com
Image credit: Vink Fan/Shutterstock.com
One particularly severe form of DNA damage is known as DNA–protein crosslinks (DPCs), in which proteins become covalently attached to DNA. DPCs can result from alcohol consumption, exposure to chemicals such as formaldehyde or other aldehydes, or from mistakes made by enzymes involved in DNA replication and repair. Because DPCs can stall DNA replication and lead to serious errors during cell division, these crosslinks represent a significant threat to genome integrity.
The enzyme SPRTN removes DPCs by cleaving DNA–protein crosslinks. Malfunction of SPRTN, for example, due to genetic mutations, may predispose individuals to bone deformities and liver cancer during adolescence. This rare genetic disorder is known as Ruijs–Aalfs syndrome. The underlying mechanisms of the disease remain poorly understood, and no specific therapies are currently available.
A research team led by Prof. Ivan Đikić from the Institute of Biochemistry II at Goethe University has now shown that the loss of a functional SPRTN enzyme not only results in the accumulation of damaged DNA in the cell nucleus. Through cell culture experiments and studies in genetically modified mice, the researchers also discovered that nuclear DNA can leak into the cytoplasm, the cell's interior.
DNA in the cytoplasm is recognized by the cell as a danger signal, since such DNA typically originates from invading viruses, bacteria, or malignant transformation. As a result, cytoplasmic DNA activates cellular defense mechanisms by triggering the cGAS–STING signaling pathway. The cell also releases messenger substances that attract immune cells, which can lead to chronic inflammation.
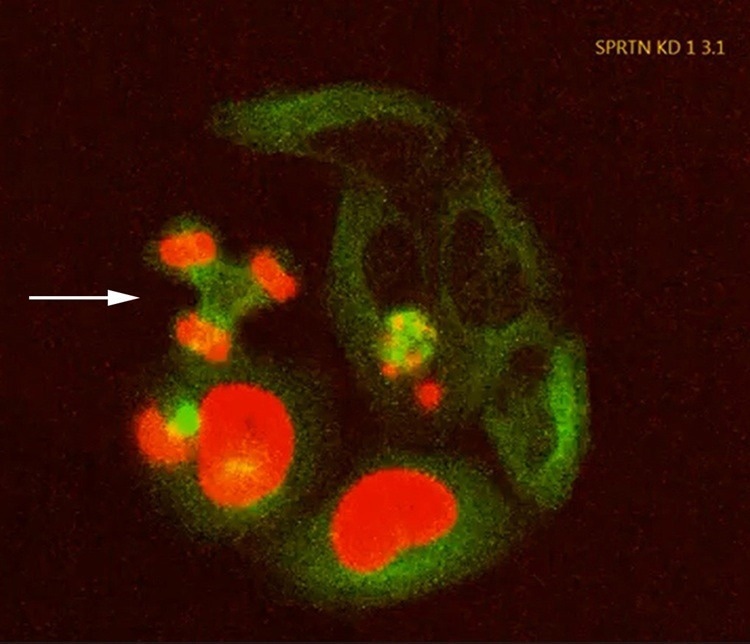
Fatal error: The failure of the repair enzyme SPRTN in these cultured cells leads to fatal errors in cell division, e.g. by distributing the chromosomes (red) to three daughter cell nuclei instead of two (arrow). Green: Cell division apparatus/cytoskeleton. Image Credit: Institute of Biochemistry II, Goethe University Frankfurt
The research team noticed that this chronic inflammatory response was particularly pronounced in mouse embryos and continued into adulthood, especially in the lung and liver. As a result, the mice died prematurely or showed signs of accelerated aging similar to those seen in individuals with Ruijs-Aalfs syndrome. Blocking the relevant immune response alleviated many of these symptoms.
Unrepaired DNA-protein crosslinks have broader systemic consequences. They not only compromise genome stability but also drive chronic inflammation that can significantly influence lifespan.
Ivan Đikić, Professor, Institute of Biochemistry II, Goethe University
The physician and molecular biologist believes this could open the door to the development of new therapies: "In addition to Ruijs-Aalfs syndrome, there are other rare genetic diseases in which DNA-protein crosslinks play an important role. With our work, we have laid an important foundation for future therapeutic approaches to these diseases as well. By studying the underlying mechanisms of these rare diseases, we discovered a new link between DNA damage, inflammatory responses, and the lifespan of an organism. This also contributes to the understanding of the biology of aging."
Source:
Journal reference:
Tomaskovic, I., et al. (2026). DNA-protein cross-links promote cGAS-STING–driven premature aging and embryonic lethality. Science. DOI: 10.1126/science.adx9445. https://www.science.org/doi/10.1126/science.adx9445.