Scientists, for the first time, developed a successful method for pinpointing proteins within different kinds of neurons in the brain of a living animal.
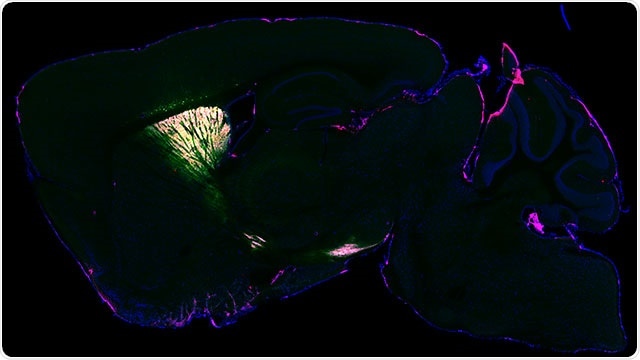
Protein expression in the mouse brain for mass spectrometry analysis, visualized by fluorescence microscopy. Blue shows the outline of the brain. Green and magenta are selectively tagged proteins. Image Credit: Northwestern University.
The research headed by Northwestern University and the University of Pittsburgh provides insight into millions of distinct proteins in the brain. Proteins, the building blocks of all cells, including neurons, hold the key to better comprehend complex brain diseases like Alzheimer’s and Parkinson’s, thus paving the way for the advancement of new treatments.
The research was published in the Nature Communications journal on August 11th, 2021.
In the current research, scientists designed a virus to transfer an enzyme to a specific location in the brain of a living mouse. The enzyme, derived from soybeans, genetically tags its neighboring proteins in a predetermined location.
After validating the process by imaging the brain with fluorescence and electron microscopy, the scientists discovered that their approach took a snapshot of the entire set of proteins (or proteome) inside living neurons, which can then be examined postmortem with mass spectroscopy.
Similar work has been done before in cellular cultures. But cells in a dish do not work the same way they do in a brain, and they don’t have the same proteins in the same places doing the same things. It’s a lot more challenging to do this work in the complex tissue of a mouse brain. Now we can take that proteomics prowess and put it into more realistic neural circuits with excellent genetic traction.”
Yevgenia Kozorovitskiy, Study Senior Author and Neurobiologist, Northwestern University
Chemically tagging proteins and their neighbors enables the scientists to determine how proteins work within a specific, controlled area and how they work with one another in a proteome. In addition to the virus carrying the soybean enzyme, the scientists also utilized their virus to carry a separate green fluorescent protein.
The virus essentially acts as a message that we deliver. In this case, the message carried this special soybean enzyme. Then, in a separate message, we sent the green fluorescent protein to show us which neurons were tagged. If the neurons are green, then we know the soybean enzyme was expressed in those neurons.”
Yevgenia Kozorovitskiy, Study Senior Author and Neurobiologist, Northwestern University
Kozorovitskiy is the Soretta and Henry Shapiro Research Professor of Molecular Biology, an associate professor of neurobiology in Northwestern’s Weinberg College of Arts and Sciences, and a member of the Chemistry of Life Processes Institute. Kozorovitskiy co-led the study with Matthew MacDonald, an assistant professor of psychiatry at the University of Pittsburgh Medical Center.
Protein targeting plays catch-up
Even when genetic targeting has fully transformed neuroscience and biology, protein targeting has unfortunately lagged. Scientists can amplify and sequence genes and RNA to pinpoint their exact building blocks.
But proteins cannot be amplified and sequenced similarly. Rather, scientists have to divide proteins into peptides and later put them back together, which is an imperfect and slow method.
“We have been able to gain a lot of traction with genetic and RNA sequencing, but proteins have been out of the loop. Yet everyone recognizes the importance of proteins. Proteins are the ultimate effectors in our cells. Understanding where proteins are, how they work, and how they work relative to each other is important,” remarks Kozorovitskiy.
Mass spectroscopy-based proteomics is a powerful technique. With our approach, we can start mapping the proteome of various brain circuits with high precision and specificity. We can even quantify them to see how many proteins are present in different parts of neurons and the brain.”
Vasin Dumrongprechachan, Study First Author and PhD Candidate, Northwestern University
Dumrongprechachan pursues his research in Kozorovitskiy’s laboratory.
Next step: Better understanding brain diseases
As the new approach is validated and is all set to go, scientists can utilize it in mouse models of disease to gain a better understanding of neurological illnesses.
“We are hoping to extend this approach to start identifying the biochemical modifications on neuronal proteins that occur during specific patterns of brain activity or with changes induced by neuroactive drugs to facilitate clinical advances,” added Dumrongprechachan.
“We look forward to taking this to models related to brain diseases and connect those studies to postmortem proteomics work in the human brain. It’s ready to be applied to those models, and we can’t wait to get started,” said Kozorovitskiy.
Source:
Journal reference:
Dumrongprechachan, V., et al. (2021) Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum. Nature Communications. doi.org/10.1038/s41467-021-25144-y.