Reviewed by Lexie CornerJun 13 2025
Researchers from the Institute for Molecular Science (IMS)/SOKENDAI and Kyushu University have identified the molecular mechanism behind the “ticking” of the circadian clock in cyanobacteria.
Their study shows how a clock protein called KaiC controls chemical reactions with precise timing. It acts like a clock hand, pausing and then moving at specific intervals.
 Visualization of phosphorylation in KaiC. Image Credit: Yoshihiko Furuike
Visualization of phosphorylation in KaiC. Image Credit: Yoshihiko Furuike
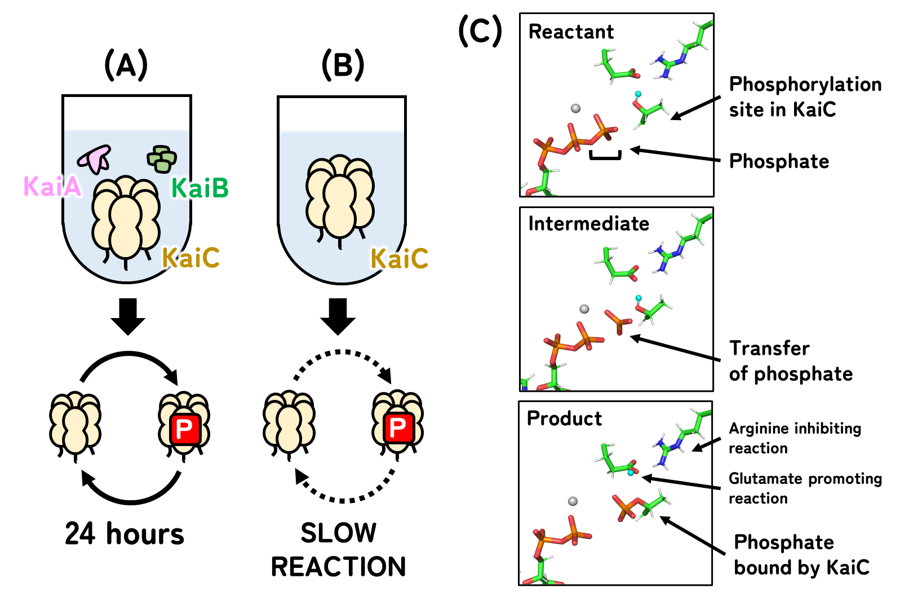
Most living organisms - from single-celled bacteria to complex mammals - have internal timekeeping systems known as circadian clocks. These clocks help them adapt to the Earth's daily rotation. Among these organisms, only cyanobacteria have a circadian clock that scientists have been able to recreate in a test tube. This system involves three proteins: KaiA, KaiB, and KaiC.
When mixed in a test tube, KaiC undergoes a regular cycle of phosphorylation and dephosphorylation - the attachment and removal of phosphate groups. This cycle repeats every 24 hours, similar to a clock (Figure 1A). However, a key question remained after years of research: how exactly does the phosphate attach to KaiC? In other words, what makes the clock hand move?
To investigate, the research team - led by Assistant Professor Yoshihiko Furuike and Professor Shuji Akiyama of IMS/SOKENDAI, and Associate Professor Toshifumi Mori of Kyushu University - focused on KaiC alone, without KaiA and KaiB. This simplified setup made it easier to observe the phosphorylation process directly. They found that KaiC can slowly bind phosphate by itself, suggesting that the core ticking mechanism resides within KaiC.
The scientists used structural data of KaiC, collected at near-atomic resolution, to simulate the phosphorylation process. They applied molecular dynamics and quantum chemical simulations to model how a phosphate group gradually approaches and binds to KaiC.
They found that small shifts in the arrangement of certain amino acids - especially glutamate and arginine - act as molecular switches. These amino acids control whether the phosphate group can attach, effectively pausing or allowing the process to continue. This means KaiC holds the clock hand steady until the correct moment for it to move.
The mechanism described in this study is not unique to cyanobacteria. Phosphorylation is also used by many organisms, including humans, to regulate their internal clocks. As a result, the findings offer broader insights into how biological timekeeping works.
Since phosphorylation is also involved in various diseases, understanding how proteins control the timing of these chemical events could inform drug development and medical research. While this study focused on phosphorylation, the related process of dephosphorylation remains poorly understood and is an important topic for future study.
Source:
Journal reference:
Furuike, Y., et al. (2025) The priming phosphorylation of KaiC is activated by the release of its autokinase autoinhibition. PNAS Nexus. doi.org/10.1093/pnasnexus/pgaf136.