Reviewed by Lauren HardakerNov 19 2025
The “blood factory” of the human body is composed of specialized tissue that includes bone cells, blood vessels, nerves, and various other cell types. Recently, researchers have achieved a significant milestone by successfully recreating this cellular complexity in the laboratory using solely human cells. This innovative system has the potential to minimize the reliance on animal experiments for numerous applications.
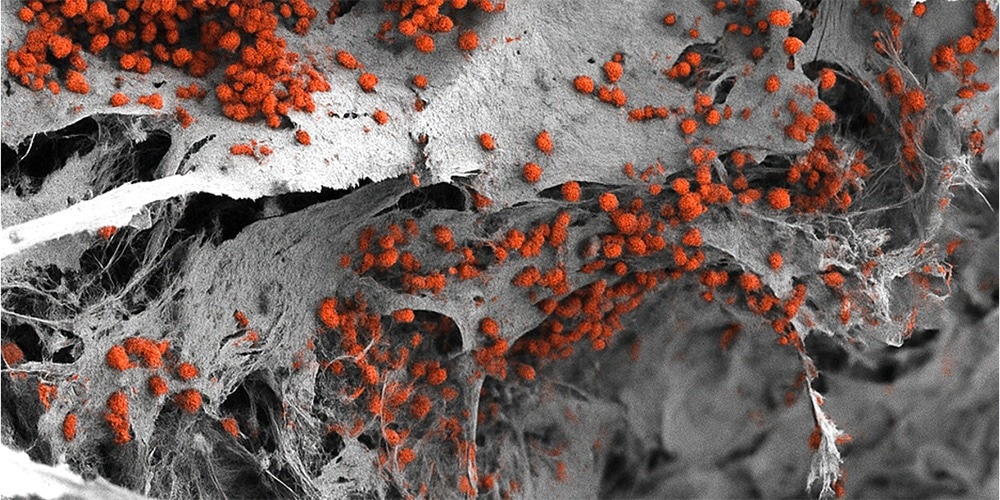 Scanning electron microscopy image of the engineered 3D bone marrow tissue colonized with human blood cells (red). Image Credit: Andrés García García, University of Basel, Department of Biomedicine
Scanning electron microscopy image of the engineered 3D bone marrow tissue colonized with human blood cells (red). Image Credit: Andrés García García, University of Basel, Department of Biomedicine
The bone marrow generally operates quietly in the background. It only becomes prominent when issues arise, such as in the case of blood cancers. In these situations, comprehending precisely how blood production functions within the human body and how this process can fail is essential.
The study on bone marrow relies significantly on animal models and overly simplified cell cultures in laboratory settings. Researchers from the Department of Biomedicine at the University of Basel and University Hospital Basel have created a realistic model of the bone marrow, constructed entirely from human cells. This model has the potential to serve as a valuable resource not only for blood cancer research but also for drug testing and possibly for personalized therapies. The research is published in Cell Stem Cell.
Bone Marrow Architecture and Cell Diversity
Bone marrow is not homogeneous; it consists of various specialized microenvironments, referred to as "niches." One niche that plays a crucial role in blood formation and is associated with the resistance of blood cancers to treatments is situated near the surface of the bone. This endosteal niche involves blood vessels, bone cells, nerves, and immune cells. To date, no human bone marrow model has incorporated all of these cellular elements.
The research team has successfully developed a model. This intricate tissue is based on an artificial bone structure composed of hydroxyapatite, which is a natural element found in bones and teeth. The team utilized human cells that were reprogrammed into pluripotent stem cells through molecular biology techniques. These artificially generated stem cells have the capability to produce various specialized cell types, contingent upon the signals they receive from their surroundings.
The researchers incorporated these stem cells into the synthetic bone framework. They directed them through particular differentiation processes to generate a diverse array of bone marrow cell types in a consistent and regulated manner.
The subsequent evaluation verified that this three-dimensional structure closely mimics the human endosteal niche and is larger than earlier systems, measuring eight mm in diameter and four mm in thickness.
The model outlined enabled the researchers to maintain human blood formation in the laboratory for several weeks.
A Step Toward Replacing Some Animal Experiments
We have learned a great deal about how bone marrow works from mouse studies. However, our model brings us closer to the biology of the human organism. It could serve as a complement to many animal experiments in the study of blood formation in both healthy and diseased conditions.
Ivan Martin, Study Corresponding Author and Professor, Department of Biomedicine, University of Basel
This aligns with the university’s efforts to replace, reduce, and refine animal experiments whenever possible.
The system could also be employed in future drug development.
However, for this specific purpose, the size of our bone marrow model might be too large.
Dr. Andrés García García, Study Corresponding Author, Department of Biomedicine, University of Basel
It is essential to miniaturize the model to evaluate various compounds and dosages simultaneously.
In the future, it is also possible to utilize the model for defining personalized treatments for blood cancers by creating individual bone marrow models from patients' cells to test various therapies and identify the most effective option for each patient. The researchers recognize that additional development will be necessary.
Source:
Journal reference:
Li, Q., et al. (2025) Macro-scale, scaffold-assisted model of the human bone marrow endosteal niche using hiPSC-vascularized osteoblastic organoids. Cell Stem Cell. DOI: 10.1016/j.stem.2025.10.009. https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(25)00377-7