Reviewed by Lauren HardakerJan 2 2026
Immune cells (macrophages) capable of combating cancer exist within tumors in the human body, but they are unable to fulfill their functions adequately due to tumor suppression. KAIST researchers have addressed this limitation by inventing a unique therapeutic strategy that directly turns immune cells within tumors into anticancer cell therapies.
 Image credit: Corona Borealis Studio/Shutterstock.com
Image credit: Corona Borealis Studio/Shutterstock.com
A research team has created a treatment in which a drug is injected directly into a tumor, absorbed by the body's existing macrophages, which then independently produce CAR (a cancer-recognizing device) proteins and transform into anticancer immune cells known as “CAR-macrophages.”
Solid tumors, such as gastric, lung, and liver cancers, form dense masses, making it harder for immune cells to penetrate and function. As a result, the efficacy of current immune cell treatments is restricted.
CAR-macrophages, which have lately gained interest as a next-generation immunotherapy, have the benefit of directly engulfing cancer cells while concurrently stimulating surrounding immune cells, hence enhancing anticancer responses.
Conventional CAR-macrophage treatments, on the other hand, need immune cells to be taken from a patient's blood before being cultured and genetically modified. This technique is time-consuming and expensive, with limited viability for real-world patient applications.
To overcome this issue, the researchers concentrated on “tumor-associated macrophages” that had already accumulated around tumors.
They devised a method to directly reprogram immune cells in the body by loading lipid nanoparticles, which are designed to be easily absorbed by macrophages, with both mRNA encoding cancer-recognition information and an immunostimulant that triggers immune responses.
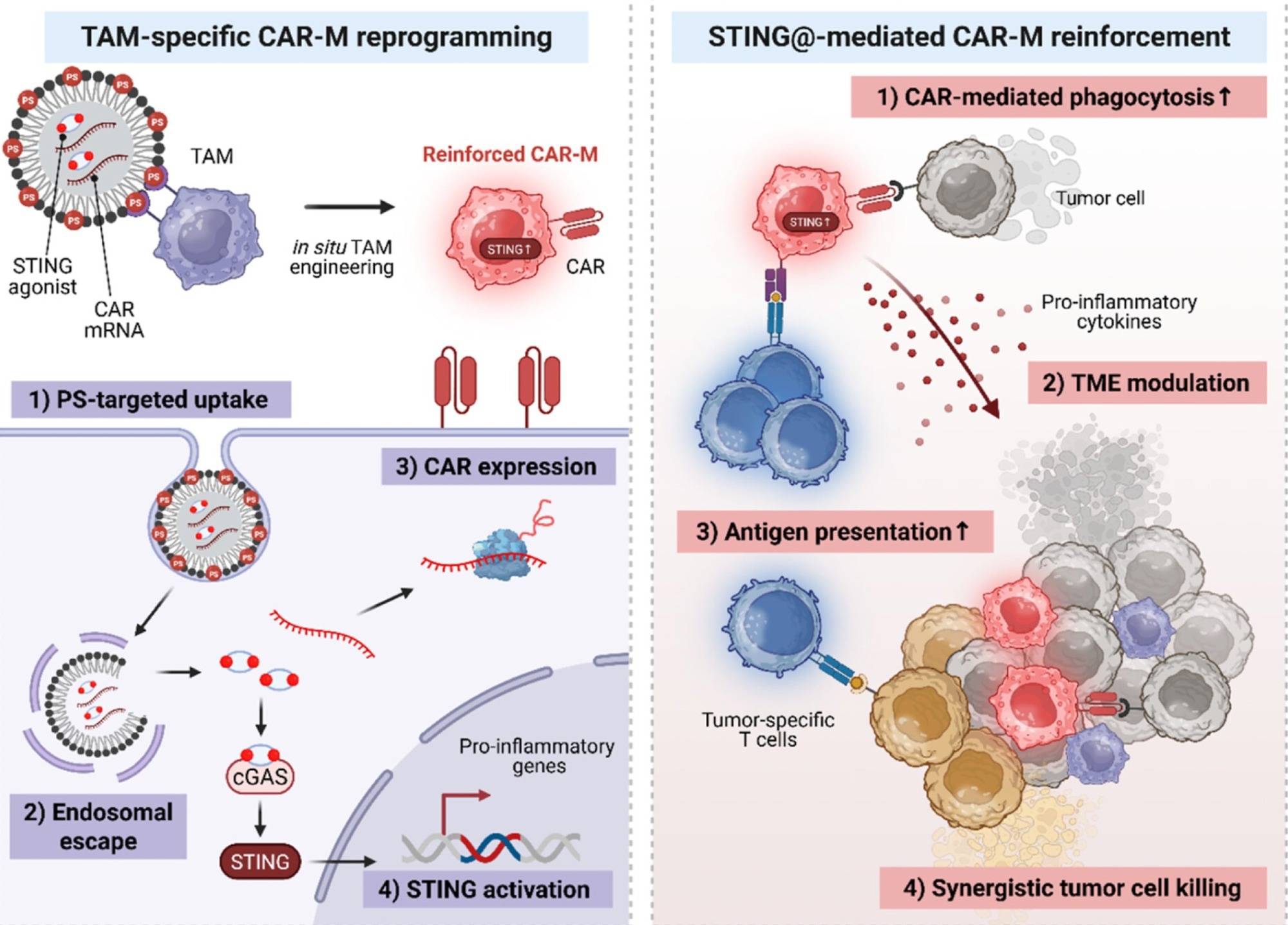 Schematic illustration of the strategy for in vivo CAR-macrophage generation and cancer cell eradication via co-delivery of CAR mRNA and immunostimulants using lipid nanoparticles (LNPs). Image Credit: Korea Advanced Institute of Science and Technology
Schematic illustration of the strategy for in vivo CAR-macrophage generation and cancer cell eradication via co-delivery of CAR mRNA and immunostimulants using lipid nanoparticles (LNPs). Image Credit: Korea Advanced Institute of Science and Technology
To put it another way, CAR-macrophages were produced in this study by “directly converting the body's own macrophages into anticancer cell therapies inside the body.”
When this therapeutic agent was injected into tumors, macrophages absorbed it quickly and began generating proteins that detect cancer cells, while immune signaling was also triggered. As a consequence, the generated "enhanced CAR-macrophages" demonstrated significantly better cancer cell killing capabilities and stimulated surrounding immune cells, resulting in a potent anticancer impact.
Tumor development was considerably inhibited in animal models of melanoma (the most severe type of skin cancer), and the therapeutic impact was demonstrated to have the ability to go beyond the local tumor site to activate systemic immune responses.
This study presents a new concept of immune cell therapy that generates anticancer immune cells directly inside the patient’s body. It is particularly meaningful in that it simultaneously overcomes the key limitations of existing CAR-macrophage therapies – delivery efficiency and the immunosuppressive tumor environment.
Professor Ji-Ho Park, Department of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology
Source:
Journal reference:
Han, J.-H., et.al. (2025) In Situ Chimeric Antigen Receptor Macrophage Therapy via Co-Delivery of mRNA and Immunostimulant. ACS Nano, 19(48), 40798–40816. DOI: 10.1021/acsnano.5c09138. https://pubs.acs.org/doi/10.1021/acsnano.5c09138.