Reviewed by Lauren HardakerFeb 16 2026
G-protein-coupled receptors (GPCRs) are one of the biggest groups of cell surface proteins in the human body, recognizing hormones, neurotransmitters, and drugs. More than 30 % of presently marketed drugs target these receptors, which regulate a wide range of physiological functions.
 Image credit: ADfoto/Shutterstock.com
Image credit: ADfoto/Shutterstock.com
The histamine H1 receptor (H1R) is a GPCR subtype that mediates allergic responses, inflammation, vascular permeability, airway constriction, wakefulness, and cognitive functioning in the human body. While antihistamines predominantly target H1R, existing drugs have poor therapeutic effectiveness, encouraging researchers to investigate H1R ligands from novel perspectives.
Recently, the significance of drug design based not only on the affinity or binding energy between a compound and its target protein, but also on its components, enthalpy, and entropy, has been recognized as critical for rational drug design.
In particular, enthalpy-entropy compensation has emerged as a critical notion for comprehending ligand selectivity and isomer specificity. However, direct experimental measurements of these thermodynamic characteristics have been confined to cell surface proteins like GPCRs.
To close this gap, a research team at Tokyo University of Science (TUS) in Japan conducted a thorough investigation of the H1R binding thermodynamics. Their findings were published online in ACS Medicinal Chemistry Letters on January 26th, 2026.
Doxepin is a compound that has been widely used as an H1R inhibitor. In this study, we successfully measured the thermodynamic signatures of doxepin geometric isomers (E- and Z-isomers) to the H1R, prepared via a budding yeast expression system, using isothermal titration calorimetry and molecular dynamics simulations.
Mitsunori Shiroishi, Professor, Department of Life System Engineering, Tokyo University of Science
Doxepin, a tricyclic antidepressant, is also a powerful antihistamine that targets H1R and comes in two isomers: E- and Z. In a recent study, the scientists found that the Z-isomer has almost five times the affinity for H1R binding than the E-isomer.
They also discovered a critical threonine residue (Thr1123.37) that contributes to the isomer-dependent selectivity. The researchers in the current work conducted a rigorous thermodynamic investigation of the H1R-doxepin interaction to explain this selectivity.
To that goal, they created two H1R variants: a wild-type (H1R_WT) variation, which they utilized in the previous investigation, and a T1123.37V mutant, in which the Thr1123.37 residue is replaced with a different amino acid. Their interaction was initially examined with doxepin (a combination of E and Z isomers), followed by individual E and Z isomers.
The results revealed no variations in binding energy for doxepin interactions between H1R_WT and the T1123.37V mutant; nevertheless, the enthalpic and entropic contributions were different. Binding to H1R_WT was mostly enthalpy-driven, whereas binding to the mutant receptor resulted in a lower enthalpic contribution complemented by a higher entropic contribution.
Notably, binding of the Z-isomer to H1R_WT resulted in a higher enthalpic gain and entropic penalty compared to the E-isomer. These changes were lacking in the T1123.37V mutant. Furthermore, for H1R_WT, the binding energy of the Z-isomer was greater than that of the E-isomer; for the mutant receptor, the binding energies of both isomers were equivalent, which is consistent with the previous study's findings.
These findings highlight the importance of Thr1123.37 in balancing enthalpic gains and entropic losses during ligand binding, as well as the stronger influence in the interaction with the Z-isomer.
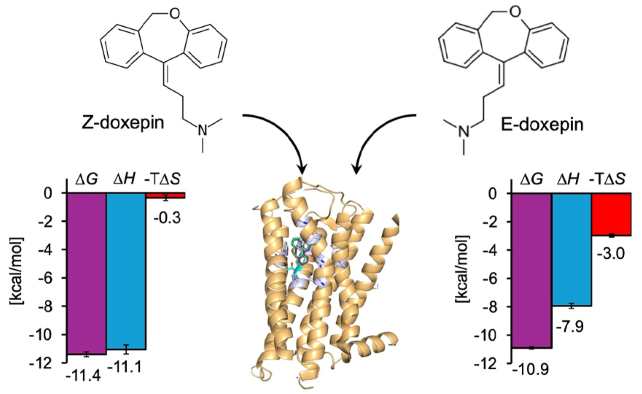 Differences in the Binding Thermodynamics of Doxepin Isomers. The findings show that considering conformational constraints and flexibility in designing ligands with optimized thermodynamic properties can lead to improved drugs with higher selectivity. Image Credit: Professor Mitsunori Shiroishi from Tokyo University of Science, Japan
Differences in the Binding Thermodynamics of Doxepin Isomers. The findings show that considering conformational constraints and flexibility in designing ligands with optimized thermodynamic properties can lead to improved drugs with higher selectivity. Image Credit: Professor Mitsunori Shiroishi from Tokyo University of Science, Japan
To better understand the molecular basis of this selectivity, the researchers ran molecular dynamics simulations, which revealed that the high-affinity binding of the Z-isomer is caused by conformational restrictions, which is consistent with the observed high enthalpy and low entropy associated with binding.
These mechanistic insights into the enthalpy-entropy trade-off in GPCR-ligand interactions highlight the importance of considering conformational constraints and flexibility in designing ligands with optimized thermodynamic properties. This could lead to the development of drugs with improved selectivity, reduced side effects, and longer-lasting therapeutic effects. Moreover, our approach, combining thermodynamic analysis with molecular dynamics simulations, can be applied to other GPCRs and proteins, aiding rational drug design.
Mitsunori Shiroishi, Professor, Department of Life System Engineering, Tokyo University of Science
The study suggests that minor molecular conformations could shift the entropy-enthalpy balance, and knowing these ideas should help designers create effective medicines while avoiding off-target effects and preserving better efficacy. Furthermore, the entropy-enthalpy balance was detected in interactions involving GPCRs as well as other proteins and drugs.
All things considered, this study gives fresh perspectives on the thermodynamic principles guiding GPCR-ligand interactions and a useful foundation for the creation of more potent therapeutics.
Source:
Journal reference:
Kaneko, H. et.al. (2026) Enthalpy–Entropy Trade-Off Underlies Geometric Isomer Selectivity in Histamine H1 Receptor–Doxepin Interaction. ACS Medicinal Chemistry Letters. Doi: 10.1021/acsmedchemlett.5c00696. https://pubs.acs.org/doi/10.1021/acsmedchemlett.5c00696.