Reviewed by Frances BriggsDec 12 2025
NMR spectroscopy reveals why LARP6 is only stable when bound to RNA.
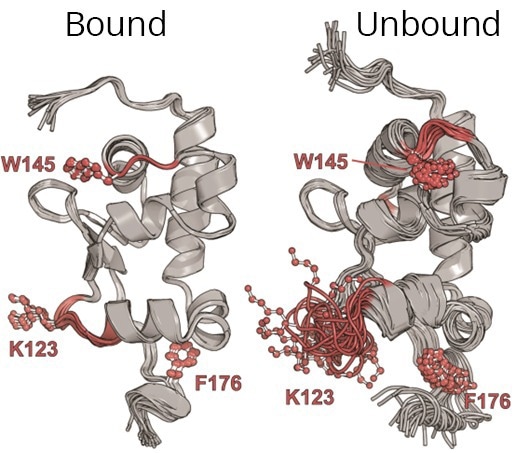 A diagram showing two versions of the Human LARP6 protein. The unbound version is not attached to RNA. The bound version is attached to an RNA, forming a tight, water-repelling core, which makes the protein more rigid and stable. Image Credit: Florida State University
A diagram showing two versions of the Human LARP6 protein. The unbound version is not attached to RNA. The bound version is attached to an RNA, forming a tight, water-repelling core, which makes the protein more rigid and stable. Image Credit: Florida State University
A research team from the Institute of Molecular Biophysics and the Department of Chemistry and Biochemistry at Florida State University has discovered an interaction between a protein found in the human body and RNA, which may lead to innovative treatments for tissue scarring, commonly referred to as fibrosis.
The researchers focused on a specific protein known as LARP6, which is instrumental in the production of type I collagen, an essential component in tissues such as skin and bones. This protein is key because of its association with conditions characterized by excessive collagen production, including fibrosis.
The team identified a novel segment of the protein that enables it to accurately recognize and bind to RNA, akin to two puzzle pieces fitting together seamlessly. This study may provide scientists with a more comprehensive understanding of how LARP6 could be targeted in therapies for diseases related to collagen overproduction. The study was published in the journal Nucleic Acids Research.
In the simplest terms, we’re trying to figure out how two molecules, just like LEGO pieces, fit together. But it’s obviously much more complicated than that, because we’re not just considering the structure of the LEGO pieces and how they fit together, but also how different parts of the LEGO pieces move around, and that all ties directly into functionality.
Robert Silvers, Principal Investigator and Assistant Professor, Department of Chemistry and Biochemistry, Florida State University
LARPs, also known as La-related proteins, constitute a superfamily of proteins found in all plants and animals. They bind to RNA, the molecule responsible for carrying genetic information, assisting in protein synthesis, and regulating DNA function.
LARP6 is one of the five primary human LARP proteins and plays a role in regulating and biosynthesizing collagen. In contrast to other LARPs, there has been minimal research conducted on the molecular interactions of LARP6 with RNA.
“Our new ‘LEGO piece’ uses a different kind of interaction with its RNA. It utilizes a different set of rules, and the protein uses a different RNA binding site altogether,” said Silvers.
The team was presented with this unique LARP by Branco Stefanovic, a professor at the FSU College of Medicine, who has dedicated a significant portion of his career to studying fibrosis. They used various techniques for observing the protein, including X-ray crystallography, before ultimately choosing NMR spectroscopy.
In NMR spectroscopy, we can look at the complex in solution close to its natural environment under physiological conditions. NMR spectroscopy is ideal as we can study the dynamics of a molecule as well as its structure.
Robert Silvers, Principal Investigator and Assistant Professor, Department of Chemistry and Biochemistry, Florida State University
NMR spectroscopy is a technique that uses the magnetic characteristics of specific nuclei to uncover the intricate structure and properties of a molecule. It has been particularly beneficial since LARP6 remains unstable until it associates with RNA.
Through the application of NMR spectroscopy, the researchers discovered that the manner in which LARP6 interacts with RNA plays a crucial role in the biosynthesis of type I collagen, a protein associated with fibrosis. This finding may assist scientists in formulating a treatment for fibrosis in the future.
Because of its function, the complex between LARP6 and RNA is something that we potentially can develop a drug for, to work against fibrosis. There is currently no drug, to my knowledge, that can slow down or stop the progression of fibrosis.
Robert Silvers, Principal Investigator and Assistant Professor, Department of Chemistry and Biochemistry, Florida State University
Source:
Journal reference:
Gordon, H. B., et al. (2025) Noncanonical RNA binding of human La-related protein 6. Nucleic Acids Research. DOI:10.1093/nar/gkaf682. https://academic.oup.com/nar/article/53/14/gkaf682/8211926?login=false