Reviewed by Lauren HardakerFeb 18 2026
Researchers at UT Southwestern Medical Center have revealed that a critical developmental process in the brain's hypothalamus might influence an individual's vulnerability to obesity.
 Image credit: T.L.F/Shutterstock.com
Image credit: T.L.F/Shutterstock.com
Their preclinical results, published in Neuron, indicate that a transcription factor called Otp functions as a molecular “switch” that drives immature hypothalamic neurons toward either appetite-suppressing or appetite-stimulating fates, determining their eventual identities as specialized cells. The researchers discovered that interrupting this switch affects eating behavior in mice and protects them against diet-induced obesity.
These findings show that early developmental decisions in the hypothalamus have a long-lasting impact on energy balance. By uncovering this fate-switching program, we can begin to understand how the brain establishes lifelong metabolic set points.
Chen Liu, PhD, Study Senior Author and Associate Professor, Internal Medicine and Neuroscience, University of Texas Southwestern Medical Center
The hypothalamic melanocortin system, which includes pro-opiomelanocortin (POMC) neurons that promote satiety (feeling full after eating) and agouti-related peptide (AgRP) neurons that cause hunger, is critical for maintaining energy balance. Although these neurons have been extensively researched in adults, it is unknown how they form during early development.
Dr. Liu and his colleagues at the Liu Lab used cutting-edge single-nucleus multiome sequencing to map the whole landscape of neurons produced from POMC-expressing precursor (parent) cells in the adult mouse hypothalamus. The researchers discovered that less than one-third of these precursor neurons continue to express POMC in adulthood. Instead, POMC precursors differentiate into several neuronal subtypes, including a large proportion of adult AgRP neurons.
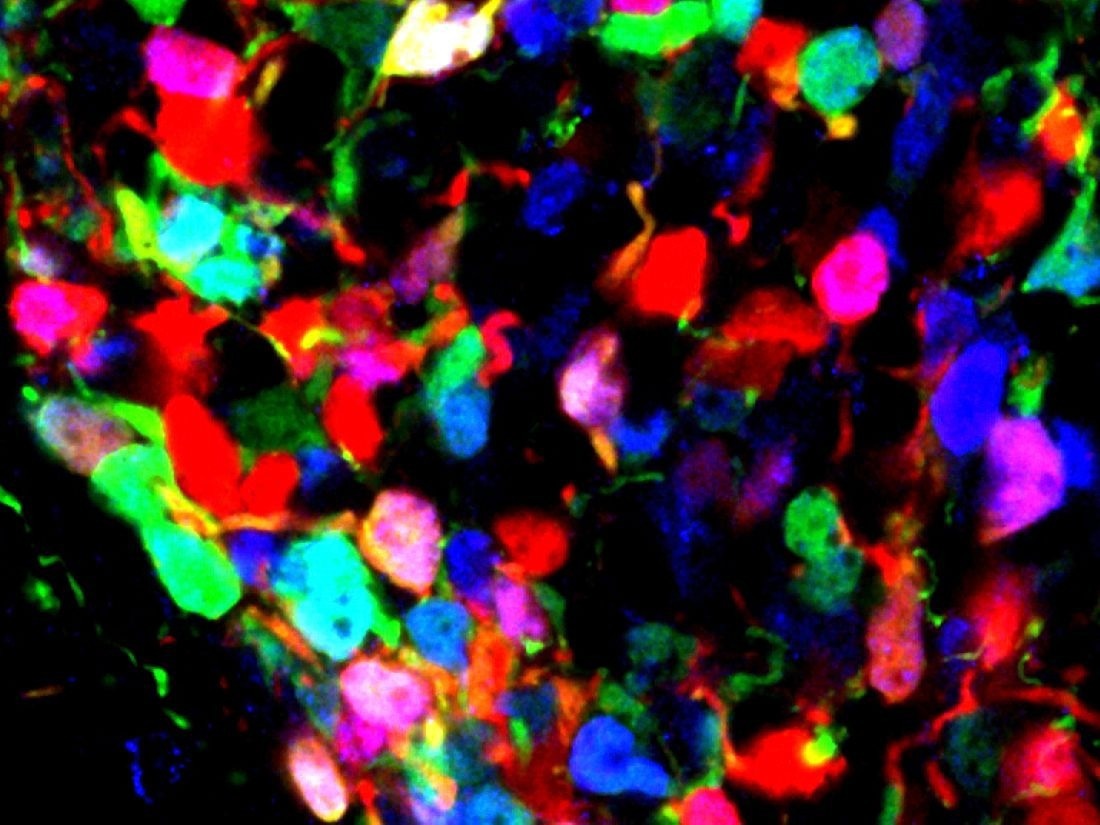 This microscopy image shows developing mouse hypothalamic tissue. Red marks POMC neuronal precursors, blue marks protein expression of the transcription factor Otp, and green marks prospective adult AgRP neurons. The image captures a developmental transition in which a subset of POMC precursors begins to express Otp as they adopt an adult AgRP neuronal identity. UT Southwestern researchers discovered that this switch may influence susceptibility to obesity. Image Credit: UT Southwestern Medical Center
This microscopy image shows developing mouse hypothalamic tissue. Red marks POMC neuronal precursors, blue marks protein expression of the transcription factor Otp, and green marks prospective adult AgRP neurons. The image captures a developmental transition in which a subset of POMC precursors begins to express Otp as they adopt an adult AgRP neuronal identity. UT Southwestern researchers discovered that this switch may influence susceptibility to obesity. Image Credit: UT Southwestern Medical Center
The study identified Otp as a critical regulator that directs POMC-derived neurons towards AgRP identity. When Otp was selectively deleted in POMC-expressing precursors, the cells did not acquire the AgRP hunger-triggering fate and instead kept alternate POMC satiety-promoting neuron identities. As a result, adult mice lacking this developmental transition had lower desires to eat high-fat diets and were less susceptible to diet-induced obesity. Females had a higher protective effect, possibly due to increased estrogen receptor (ERα) signaling in specific POMC-derived subpopulations.
From an evolutionary standpoint, the POMC→AgRP fate switch likely served as an adaptive mechanism. In environments where food availability fluctuated, animals needed a rapid, robust way to increase food intake when high-calorie food became available. By generating a population of highly responsive ‘hunger’ neurons, this developmental switch enabled overeating, helping animals build energy reserves and survive periods of scarcity.
Chen Liu, PhD, Study Senior Author and Associate Professor, Internal Medicine and Neuroscience, University of Texas Southwestern Medical Center
In today's world, when calorie-dense foods are more easily available, Dr. Liu believes that this once-beneficial strategy might increase vulnerability to obesity. The team's results show that turning off this switch during early development protects the brain from responding to high-fat meals, decreasing obesity risk. He explained that this disparity reflects a broader pattern in metabolic disease, where biological mechanisms that evolved to support survival in ancestral environments may function differently in modern contexts.
Dr. Liu stated that he and his colleagues intend to examine whether external variables, such as maternal overnutrition or undernutrition, influence this genetic fate-switch mechanism and so alter metabolic health later in life.
Source:
Journal reference:
Xu, B. et.al. (2026) Developmental reprogramming in melanocortin neurons modulates diet-induced obesity in mice. Neuron. DOI: 10.1016/j.neuron.2025.12.022. https://www.cell.com/neuron/fulltext/S0896-6273(25)00978-X.