Reviewed by Lauren HardakerNov 24 2025
Every cell within an organism shares an identical genetic blueprint. Their distinct identities stem from epigenetics, precise chemical markers that dictate gene expression. Errors in this epigenetic regulation can result in significant developmental issues in both plants and animals. This naturally leads to a fundamental query: what controls these epigenetic modifications if they, in turn, regulate human genes?

Image credit: Corona Borealis Studio/Shutterstock.com
Using plant cells, scientists at the Salk Institute have now uncovered that DNA methylation, a key epigenetic tag, can be controlled through genetic mechanisms. This newly identified targeting method for plant DNA methylation relies on specific DNA sequences to direct the methylation machinery to its precise location.
The scientific community understood DNA methylation regulation primarily through other epigenetic features. Therefore, understanding that genetic elements can also steer DNA methylation patterns represents a significant shift in their understanding.
These findings could guide the development of future epigenetic engineering strategies. Such strategies would be designed to establish methylation patterns predicted to restore or improve cell function, offering numerous potential applications in medicine and agriculture.
In plants and animals, incorrect patterns of DNA methylation can cause developmental defects, and in mammals, that can lead to numerous diseases – including cancer. This makes it very important for us to understand how DNA methylation is targeted to the correct locations in the correct tissues and developmental stages. Our work answers a long-standing question about how new patterns of methylation are generated during plant development, which is the first step in thinking about engineering DNA methylation patterns to improve cellular fitness.
Julie Law, Ph.D., Study Senior Author, Biochemist and Associate Professor, Salk Institute
What is Epigenetics?
Cellular blueprints are carefully made using a four-letter alphabet: A, T, C, and G. These combine to form the extensive chains of DNA. These long, intricate DNA strands are then carefully wound around specialized proteins called histones, forming a compact structure known as chromatin.
This packaging system efficiently condenses and organizes the genetic material, ensuring both its secure storage and ready accessibility. The epigenome is a dynamic layer of molecular tags and modifications layered onto the DNA. These epigenetic changes precisely control which genes are activated or silenced, all without altering the underlying DNA sequence itself, thus providing essential flexibility in how cells define themselves and behave.
A key player in this epigenetic regulation is DNA methylation, a process where a methyl group is chemically attached to specific "C" (cytosine) bases within the DNA code. These DNA methylation tags act as signals to switch off the associated DNA, a mechanism referred to as "silencing."
This silencing process is critically important, not only for regulating the normal expression of genes but also for suppressing the activity of unique genetic elements known as transposons. If allowed to express, transposons can relocate within the genome, potentially leading to genomic instability and a decline in an organism's overall fitness.
Developing a comprehensive understanding of how, when, and why specific DNA methylation patterns are established in different cell types is absolutely vital. This knowledge is indispensable for deciphering the intricacies of biological development and for creating targeted therapies for diseases that stem from epigenetic dysfunction.
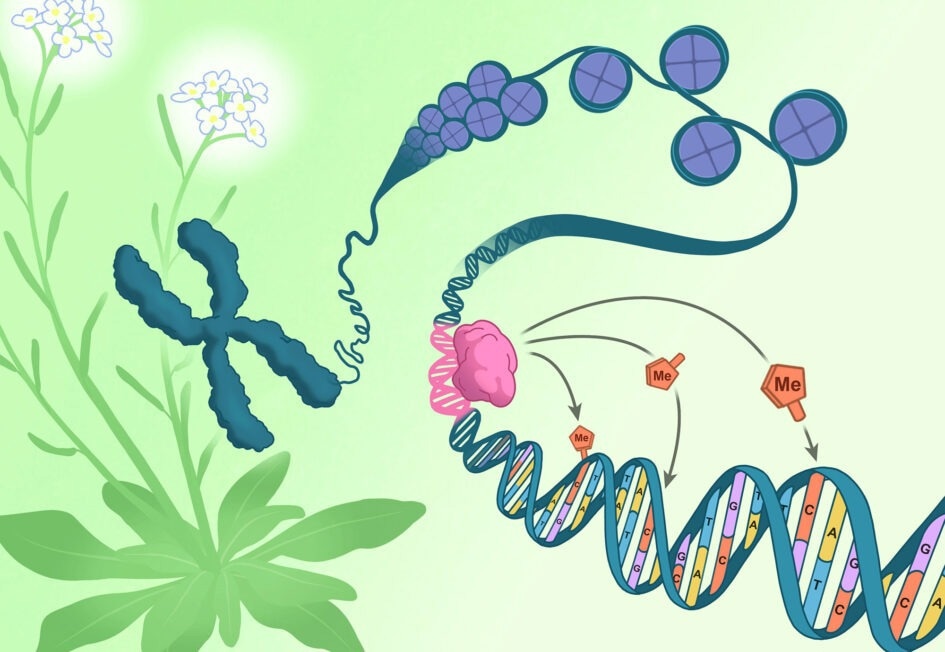 A chromosome pulled from the flowers of Arabidopsis thaliana (green and white) unspools to reveal DNA (blue) coiled around packaging-proteins called histones (purple). The direction of epigenetic changes by genetic features begins as the RIM transcription factor (pink) docks on a corresponding DNA sequence (pink). Once docked, the RIM transcription factor directs methylation machinery to tack methyl groups (orange) onto specific nearby cytosines (orange). Image Credit: Salk Institute
A chromosome pulled from the flowers of Arabidopsis thaliana (green and white) unspools to reveal DNA (blue) coiled around packaging-proteins called histones (purple). The direction of epigenetic changes by genetic features begins as the RIM transcription factor (pink) docks on a corresponding DNA sequence (pink). Once docked, the RIM transcription factor directs methylation machinery to tack methyl groups (orange) onto specific nearby cytosines (orange). Image Credit: Salk Institute
We’ve learned a lot about how an epigenetic tag can be maintained after it’s been established. But cellular diversity doesn’t come from sustained patterns; it comes from new patterns, and there’s a lot we still don’t know about what creates a new epigenetic pattern. This work is filling that gap between knowing epigenetic diversity exists and understanding how it is generated.
Julie Law, Ph.D., Study Senior Author, Biochemist and Associate Professor, Salk Institute
Why Study Epigenetics in Plants?
Arabidopsis thaliana, a very small flowering plant, has been a model organism in laboratories for many years. Its resilience to experimental alterations in epigenetic modifications, surpassing that of human or animal cells, makes it an invaluable tool for exploring core epigenetic principles.
Within Arabidopsis, the arrangement of DNA methylation is governed by four proteins known as the CLASSYs. Individually, each CLASSY protein directs the DNA methylation apparatus to specific sites across the genome. However, before the recent Salk investigation, researchers lacked understanding regarding the mechanism by which CLASSY3 achieved this precise targeting. The specific factors influencing its selection of particular genomic regions over others remained a mystery.
How do Epigenetic Changes Start?
Understanding DNA methylation has traditionally focused on how it is maintained, with existing epigenetic marks guiding where methylation is added. A well-known example is the restoration of methylation at a specific genomic site following cell division, which preserves the repression of gene expression in that region.
The stability provided by these self-reinforcing mechanisms is crucial for maintaining epigenetic patterns throughout an organism's lifespan. For instance, during the division of a mature skin cell into two daughter cells, maintaining the existing epigenetic blueprint is essential to prevent the spontaneous emergence of new patterns that could potentially lead to cellular dysfunction or oncogenesis.
However, a critical question arises concerning scenarios where epigenetic patterns must change, such as during developmental processes or in adaptation to environmental stressors. Specifically, how does a plant cell dynamically adjust its epigenome to allow growth, mount appropriate responses, and ensure recovery?
How do these patterns start? We wanted to know what was regulating epigenetic pathways to create new DNA methylation patterns during plant development, regeneration, and reproduction.
Guanghui Xu, Ph.D., Study First Author and Postdoctoral Researcher, Salk Institute
A Paradigm Shift in Plant DNA Methylation
To understand how specific DNA methylation patterns emerge, scientists focused their investigation on the reproductive tissues of Arabidopsis plants. They uncovered a new method of DNA methylation targeting, using a forward genetic screening approach. This approach is dictated by DNA sequences themselves rather than existing epigenetic factors.
The research team identified several proteins, which they named “RIMs,” that work with CLASSY3 to establish DNA methylation at precise genomic locations within plant reproductive tissues.
These RIMs are categorized as a subset of the extensive REPRODUCTIVE MERISTEM (REM) transcription factor protein family. This discovery was particularly surprising as it directly linked CLASSY3's targeting mechanism to specific DNA sequences. When these critical DNA stretches were experimentally disrupted, the entire methylation pathway failed to function.
This study pinpoints crucial DNA segments where RIMs attach, subsequently enabling them to direct the DNA methylation machinery to modify nearby DNA sequences. Through this targeted action, the researchers demonstrated that unique methylation patterns are generated in reproductive tissues, varying based on the different combinations of RIMs expressed.
This represents the first instance where scientists have identified a genetic sequence that can directly influence the epigenetic process of DNA methylation in plants. Given the numerous REM genes present in Arabidopsis, the team anticipates that additional members of this family will also be found to play roles in DNA methylation, thereby expanding their known functions in controlling epigenetic regulation.
Further supporting the idea that genetic information guides epigenetic processes, another study employed reverse genetics to identify several REM genes involved in regulating DNA methylation through specific DNA sequences.
This finding represents a paradigm shift in the field’s view of how methylation is regulated in plants. All previous work pointed to pre-existing epigenetic modifications as the starting place for targeting methylation, which didn’t explain how novel methylation patterns could arise. Now we know the DNA itself can instruct new methylation patterns, too.
Julie Law, Ph.D., Study Senior Author, Biochemist and Associate Professor, Salk Institute
This finding, that genetic features can influence epigenetic modifications, creates many new opportunities for investigation. Researchers are especially interested in how widespread this targeting mechanism is during plant development and in exploring ways it could be harnessed to engineer precise DNA methylation patterns.
The capacity to use DNA sequences for targeted methylation holds profound implications for both agriculture and human health, as it would enable the highly precise correction of epigenetic defects.
Source:
Journal reference:
Xu, G., et al. (2025) Transcription factors instruct DNA methylation patterns in plant reproductive tissues. Nature Cell Biology. DOI:10.1038/s41556-025-01808-5. https://www.nature.com/articles/s41556-025-01808-5